Abstract
The cause and effect relationship between serum uric acid levels and hypertension can be difficult to evaluate because antihypertensive drugs sometimes affect uric acid levels. This cross-sectional study investigated the relationship between serum uric acid levels and hypertension in a general, healthy Japanese population who were not receiving medication for hyperuricemia or hypertension. We retrospectively analyzed the medical records of 90 143 Japanese people (men, 49.1%; age, 46.3±12.0 years) undergoing an annual medical examination at St Luke’s International Hospital Center for Preventive Medicine, Tokyo, between January 2004 and June 2010. Of these individuals, 82 722 (91.8%) who had never taken medications for gout, hyperuricemia or hypertension were enrolled. We compared the participant characteristics and prevalence of diastolic hypertension (⩾90 mm Hg) and/or systolic hypertension (⩾140 mm Hg) by serum uric acid quartile. The odds ratio (OR) of hypertension was 1.20 for each 1 mg dl−1 increase in serum uric acid level after adjustment for age, sex, body mass index (BMI), dyslipidemia, diabetes, smoking and estimated glomerular filtration rate (eGFR). Compared with the lowest serum uric acid quartile, participants in the highest quartile had a 3.7-fold higher OR for hypertension. After adjustment for age, BMI, dyslipidemia, diabetes, smoking and eGFR, these ORs were 1.79 (1.62–1.98) in the total study population, 1.58 (1.44–1.75) in men and 1.60 (1.39–1.84) in women. The results were similar for both systolic and diastolic hypertension. Elevated serum uric acid levels may be as important as obesity, dyslipidemia, diabetes, smoking and reduced kidney function for the development of hypertension and should be considered in hypertension prevention programs.
Similar content being viewed by others
Introduction
Hyperuricemia is frequently associated with lifestyle-related diseases.1, 2 Approximately 25–40% of untreated hypertensive patients have concomitant hyperuricemia,3, 4 and an association between elevated serum uric acid (SUA) and hypertension (HT) has been described in adults in several large epidemiological studies.5, 6, 7, 8, 9, 10, 11 Hyperuricemia may be an independent risk factor for cardiovascular events as well as for metabolic syndrome, gout and renal failure. Although hyperuricemia is known to be correlated with cardiovascular events in hypertensive patients,5, 6, 10 it remains unknown whether uric acid is an independent risk factor, a mediator or merely a marker for the development of HT.12 Experimental data suggest that uric acid may induce endothelial damage, vascular inflammation and renin–angiotensin system activation.13, 14
Several recent small clinical trials have demonstrated that SUA-lowering agents such as allopurinol and probenecid can reduce blood pressure (BP) in adolescents,15, 16 suggesting that UA is an independent risk factor for the development of HT. Other reports, including the PIUMA,5 SHEP,6 LIFE7 and Framingham studies,8, 11 have demonstrated an association between high levels of SUA and cardiovascular diseases in HT patients. However, these observations were made in hypertensive patients who were treated with antihypertensive agents that have an influence on SUA levels. Losartan and long-acting calcium antagonists usually decrease SUA levels and reduce the risk of developing gout. Thiazide diuretics, β-blockers, angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists often increase SUA levels and may induce a gout attack.17 Thus far, few reports have explored the relationship between SUA levels and HT in an untreated, healthy general population. Therefore, we conducted an epidemiological study to examine the relationship between SUA levels and HT in generally healthy people who were not receiving medication for gout, hyperuricemia or HT.
Methods
This study was a large-scale, single-center, cross-sectional study. We retrospectively analyzed the medical records of 90 143 subjects (men, 49.1%; age, 46.3±12.0 years) who underwent an annual medical examination at St Luke’s International Hospital Center for Preventive Medicine, Tokyo, Japan, between January 2004 and June 2010.
BP readings were obtained using an automatic brachial sphygmomanometer (OMRON Corporation, Kyoto, Japan). Two readings were taken after the participant had been seated and resting quietly for >5 min with the feet on the ground and the back supported. The mean systolic and diastolic BP for each participant were calculated from the recorded measurements. Systolic HT was defined as a systolic BP of ⩾140 mm Hg, and diastolic HT was defined as a diastolic BP of ⩾90 mm Hg. Participants with systolic and/or diastolic HT constituted the HT group.
We divided the study population by quartiles according to the SUA level and calculated the prevalence of diastolic and systolic HT in each quartile. Analysis of variance with Tukey’s post hoc method was used to determine significant differences between quartiles. We adjusted our analysis for age, body mass index (BMI), dyslipidemia, diabetes, smoking and estimated glomerular filtration rate (eGFR). Dyslipidemia was defined as a low-density lipoprotein cholesterol level of ⩾140 mg dl−1, a high-density lipoprotein cholesterol level of <40 mg dl−1 or a triglyceride level of ⩾150 mg dl−1. Diabetes was defined as an HbA1c concentration of ⩾6.1% (as per the Japanese Diabetes Society). The dyslipidemia and diabetes groups included patients who were followed up and were receiving medication for these diseases.
Statistical analysis
Statistical analyses were performed using SPSS Statistics software (IBM SPSS Statistics version 19 for Windows; IBM, New York, NY, USA). Statistical significance was set at α=0.05. All the statistical analyses were two sided. Bivariate associations between demographic and clinical characteristics were compared in HT and normal BP groups using t-tests and χ2 analyses. The characteristics of the participants were compared between SUA quartiles in a similar way. Significant differences among the quartiles were evaluated using simple regression of the clinical variable on the SUA quartile; the lowest quartile served as the reference group.
The association of SUA level with HT was evaluated by logistic regression. All regression analyses were adjusted for age, sex, BMI, dyslipidemia, diabetes, smoking and eGFR. Because the distribution of SUA levels differed between men and women, multivariate regression analyses were also stratified by gender. Logistic regression analyses modeled the SUA level in two ways: as a continuous variable in mg dl−1 and by quartiles, with the lowest quartile serving as the reference group.
Results
Of the 90 143 subjects, 82 722 (91.8%) met the criteria for enrollment in this study. Five subjects were excluded because of lack of blood test data, and two were excluded because no BP data were available. Patients receiving medication for gout (7414, 8.2%), hyperuricemia (1884, 2.1%) and/or HT (6199, 6.9%) were also excluded. An additional 7480 participants (9.0%) with HT were not taking any medication for the disease. The regression analyses were performed in the overall group, and there were significant differences between HT and normal BP groups with respect to age, sex, weight, height, BMI, eGFR, SUA and prevalence of smoking, dyslipidemia and diabetes (P<0.001; Table 1).
The mean SUA was 5.90 mg dl−1 in the HT group and 5.15 mg dl−1 in the normal BP group (P<0.001) and was significantly higher in men (6.47 mg dl−1 in the HT group, 6.15 mg dl−1 in the normal BP group) than in women (4.87 mg dl−1 in the HT group, 4.32 mg dl−1 in the normal BP group; Figure 1).
Mean serum uric acid in normal blood pressure and hypertension groups. *Significant difference between normal blood pressure and hypertension (P<0.001). **Significant difference between men and women. (P<0.001). A full color version of this figure is available at the Hypertension Research journal online.
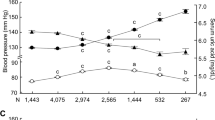
Odds ratios (ORs) and 95% confidence intervals (CIs) for age (per 1 year increase), sex (women vs. men), BMI (per 1 kg m−2 increase), dyslipidemia (negative vs. positive), diabetes (negative vs. positive), smoking (nonsmoker vs. smoker), eGFR (per 1 ml min−1 per 1.73 m2 increase) were 1.07 (95% CI: 1.07–1.07), 1.21 (95% CI: 1.13–1.30), 1.21 (95% CI: 1.20–1.22), 1.20 (95% CI: 1.14–1.27), 1.20 (95% CI: 1.08–1.32), 1.27 (95% CI: 1.19–1.33) and 1.01 (95% CI: 1.01–1.01), respectively. After adjustment for age, sex, BMI, dyslipidemia, diabetes, smoking and eGFR, the OR of HT for each 1 mg dl−1 increase in the SUA level was 1.20 (95% CI: 1.18–1.23; Table 2). Compared with participants in the lowest SUA quartile, those in the highest quartile had 3.7-fold higher odds of HT (Figure 2). We calculated each SUA quartile of each gender to account for gender differences in SUA. In male subjects, participants in the highest SUA quartile had 1.7-fold higher odds of HT than those in the lowest quartile. Women in the highest SUA quartile had 3.4-fold higher odds of HT than those in the lowest quartile (Figure 2). After adjustment for age, BMI, dyslipidemia, diabetes, smoking and eGFR, these ORs were 1.79 (95% CI: 1.62–1.98) in the total study population, 1.58 (95% CI: 1.44–1.75) in men and 1.60 (95% CI: 1.39–1.84) in women (Table 3). The results were similar for both systolic and diastolic HT (Table 4).
Prevalence of hypertension by serum uric acid quartile. Quartile ranges: (a) overall: 1 (0.1–4.1 mg dl−1), 2 (4.2–5.0 mg dl−1), 3 (5.1–6.1 mg dl−1) and 4 (6.2–12.8 mg dl−1). (b) Men: 1 (0.1–5.3 mg dl−1), 2 (5.4–6.1 mg dl−1), 3 (6.2–6.9 mg dl−1) and 4 (7.0 mg dl−1). (c) Women: 1 (0.3–3.6 mg dl−1), 2 (3.7–4.2 mg dl−1), 3 (4.3–4.8 mg dl−1) and 4 (4.9 mg l−1). *Significant difference between groups (P<0.001 between quartiles by analysis of variance (ANOVA) with Tukey’s post hoc method).
The mean BP was significantly higher in men (120.6/75.3 mm Hg) than in women (111.3/68.9 mm Hg). Systolic and diastolic BP were both significantly higher when the SUA level was elevated. These trends were similar when participants were stratified by sex (Table 5).
Discussion
This epidemiological study was large in scale and involved high-quality data acquisition because of the very low exclusion rate, with deficit data of only seven (0.00%). In the present study, the OR of HT was 1.20 for each 1 mg dl−1 increase in the SUA level after multiple adjustments.
Although several reports have suggested that SUA has an independent relationship with HT,3, 9, 16, 18 SUA elevation in HT could be a consequence of hyperinsulinemia and reduced renal function,19 and metabolic syndrome is a potential confounder of the relationship between SUA and HT. Insulin resistance and resultant hyperinsulinemia are thought to play an important role in the pathophysiology of metabolic syndrome.20 Insulin is known to decrease the renal excretion of uric acid,21, 22 and hyperinsulinemia is a predisposing factor for the development of HT.23 Imazu et al.24 reported that the relationship between SUA and the development of HT is independent of the insulin level; however, we did not measure the serum insulin level in our study. Hyperinsulinemia and components of metabolic syndrome are reported to be associated with the SUA level. When we adjusted for several elements of metabolic syndrome, including BMI, dyslipidemia and diabetes, SUA remained a significant predictor of HT. We also found that higher SUA levels predispose individuals to the development of HT, independent of renal function, as assessed by eGFR. An elevated SUA level is associated with decreased renal perfusion, decreased tubular secretion of uric acid and activation of the renin–angiotensin system.14, 25, 26
Because some subjects in the present study exhibited renal dysfunction, our results may have been influenced by this impaired renal function. In addition, several studies have indicated that uric acid can induce endothelial damage and vascular inflammation and can activate the renin–angiotensin system.13, 14 The mechanism by which hyperuricemia causes HT has been investigated in animal models. It has been suggested that uric acid induces acute vasoconstriction via activation of the renin–angiotensin system, followed by uric acid uptake into vascular smooth muscle cells. This process then leads to cellular proliferation and secondary arteriolosclerosis that impairs pressure natriuresis.26 Soletsky and Feig16 demonstrated that treatment with either allopurinol or probenecid reduced BP in adolescent subjects, and our results support this observation in adult subjects. Taken together, these data suggest that SUA could be an independent association factor for the development of HT in the healthy general population.
The clinical implications of the elevated SUA levels in hypertensive patients remain to be elucidated. Antihypertensive drugs such as thiazide raise the SUA level. Choi et al.17 reported that gout is likely to occur with administration of antihypertensive drugs that elevate the SUA level. In the present study, we found that patients with HT often exhibited hyperuricemia as a comorbidity even if they were not taking medication. Thus, it is important to monitor the SUA level in hypertensive patients regardless of whether antihypertensive agents are administered.
The identification of SUA as an independent association factor for the development of HT, irrespective of gender, was our most significant finding. SUA is known to be higher in men compared with women. Sex hormones such as estrogen and progesterone increase renal uric acid excretion by inhibiting the function of the uric acid transporter.27 Several reports have demonstrated that men have higher odds of an elevated BP with increasing SUA levels as compared with women.9, 28 However, we found that both genders had significantly higher odds of an elevated BP with increasing SUA. These findings indicate that an elevated SUA level is an association factor for the development of HT in both men and women.
There were several limitations in the present study, including the retrospective, cross-sectional study design, the possible selection bias because information was only obtained from individuals who underwent an annual medical examination at a single center and the limited novelty (similar results were reported in a non-Japanese population). Nonetheless, it is noteworthy that this study clarified a close relationship between SUA and HT not only in men but also in women in a general, healthy Japanese population.
We must seriously consider the possible existence of confounding factors. For example, factors such as obesity or a type A personality may cause both HT and hyperuricemia. Logistic regression analysis revealed significant relationships between HT and the following parameters: age (OR: 1.07 per 1-year increase), male gender (OR: 1.21), BMI (OR: 1.21 per 1 kg m−2 increase), dyslipidemia (OR: 1.20), diabetes (OR: 1.20), smoking (OR: 1.27) and eGFR (OR: 1.01 per 1 ml min−1 per 1.73 m2 increase). Information on personality type, such as type A, was not available. Because we only measured BP at the outpatient clinic, it is possible that some HT patients might have white-coat HT and that some non-HT patients might have home HT. Ambulatory BP monitoring may be the best method for BP evaluation; however, this is difficult in practice in the setting of an annual medical examination.
References
Roddy E . Hyperuricemia, gout, and lifestyle factors. J Rheumatol 2008; 35: 1689–1691.
Choi HK . A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol 2010; 22: 165–172.
Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH . Hyperuricemia in primary and renal hypertension. New Engl J Med 1966; 275: 457–464.
Gois PH, Souza ER . Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst Rev 2013; 1: CD008652.
Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P . Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000; 36: 1072–1078.
Franse LV, Pahor M, Di Bari M, Wan JY, Somes GW, Applegate WB . Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP). J Hypertens 2000; 18: 1149–1154.
Hoieggen A, Alderman MH, Kjeldsen SE, Julius S, Devereux RB, De Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Chen C, Dahlöf B, LIFE Study Group. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int 2004; 65: 1041–1049.
Culleton BF, Larson MG, Kannel WB, Levy D . Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999; 131: 7–13.
Loeffler LF, Navas-Acien A, Brady TM, Miller ER 3rd, Fadrowski JJ . Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey, 1999-2006. Hypertension 2012; 59: 811–817.
Alderman MH, Cohen H, Madhavan S, Kivlighn S . Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 1999; 34: 144–150.
Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS . Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005; 45: 28–33.
Johnson RJ, Sanchez-Lozada LG, Mazzali M, Feig DI, Kanbay M, Sautin YY . What are the key arguments against uric Acid as a true risk factor for hypertension? Hypertension 2013; 61: 948–951.
Kanellis J, Kang DH . Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol 2005; 25: 39–42.
Feig DI, Kang DH, Johnson RJ . Uric acid and cardiovascular risk. New Engl J Med 2008; 359: 1811–1821.
Feig DI, Soletsky B, Johnson RJ . Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008; 300: 924–932.
Soletsky B, Feig DI . Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 2012; 60: 1148–1156.
Choi HK, Soriano LC, Zhang Y, Rodriguez LA . Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ 2012; 344: d8190.
Kostka-Jeziorny K, Uruski P, Tykarski A . Effect of allopurinol on blood pressure and aortic compliance in hypertensive patients. Blood Press 2011; 20: 104–110.
Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG . Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Ann Intern Med 1980; 93: 817–821.
Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH . The metabolic syndrome. Endocr Rev 2008; 29: 777–822.
Quinones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, Ferrannini E . Effect of insulin on uric acid excretion in humans. Am J Physiol 1995; 268 (1 Pt 1): E1–E5.
Muscelli E, Natali A, Bianchi S, Bigazzi R, Galvan AQ, Sironi AM, Frascerra S, Ciociaro D, Ferrannini E . Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens 1996; 9: 746–752.
Sowers JR, Khoury S, Standley P, Zemel P, Zemel M . Mechanisms of hypertension in diabetes. Am J Hypertens 1991; 4 (2 Pt 1): 177–182.
Imazu M, Yamamoto H, Toyofuku M, Sumii K, Okubo M, Egusa G, Yamakido M, Kohno N . Hyperinsulinemia for the development of hypertension: data from the Hawaii-Los Angeles-Hiroshima Study. Hypertens Res 2001; 24: 531–536.
Sanchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodríguez-Iturbe B, Johnson RJ . Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol 2008; 295: F1134–F1141.
Feig DI . Hyperuricemia and hypertension. Adv Chronic Kidney Dis 2012; 19: 377–385.
Takiue Y, Hosoyamada M, Kimura M, Saito H . The effect of female hormones upon urate transport systems in the mouse kidney. Nucleosides Nucleotides Nucleic Acids 2011; 30: 113–119.
Gaffo AL, Jacobs DR Jr., Sijtsma F, Lewis CE, Mikuls TR, Saag KG . Serum urate association with hypertension in young adults: analysis from the Coronary Artery Risk Development in Young Adults cohort. Ann Rheum Dis Sep 14 2012; 72: 1321–1327.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuwabara, M., Niwa, K., Nishi, Y. et al. Relationship between serum uric acid levels and hypertension among Japanese individuals not treated for hyperuricemia and hypertension. Hypertens Res 37, 785–789 (2014). https://doi.org/10.1038/hr.2014.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.75
Keywords
This article is cited by
-
Hip circumference has independent association with the risk of hyperuricemia in middle-aged but not in older male patients with type 2 diabetes mellitus
Nutrition Journal (2023)
-
Update in uric acid, hypertension, and cardiovascular diseases
Hypertension Research (2023)
-
Mediating effects of insulin resistance on the development of hypertension associated with elevated serum uric acid: a prospective cohort study
Journal of Human Hypertension (2022)
-
Association between metabolic syndrome and uric acid: a systematic review and meta-analysis
Scientific Reports (2022)
-
Uric Acid, Hypertensive Phenotypes, and Organ Damage: Data from the Pamela Study
Current Hypertension Reports (2022)