Abstract
Hypertension promotes and escalates kidney injury, including kidney fibrosis. Fractalkine/CX3CL1 is a unique chemokine that works as a leukocyte chemoattractant and an adhesion molecule. Recently, fractalkine/CX3CL1 has been reported to promote tissue fibrosis via its cognate receptor, CX3CR1. However, the involvement of the fractalkine-CX3CR1 axis in the pathogenesis of hypertensive kidney fibrosis remains unclear. The impacts of the fractalkine-CX3CR1 axis on hypertensive kidney fibrosis were investigated in a deoxycorticosterone acetate (DOCA)-salt hypertensive model in CX3CR1-deficient mice, which were sacrificed on day 28. The blood pressure levels were similarly elevated in both CX3CR1−/− C57BL/6 and wild-type C57BL/6 mice. Fractalkine and CX3CR1 were upregulated in kidneys that were damaged by hypertension. Deficiency in CX3CR1 inhibited kidney fibrosis, as evidenced by a decrease in the presence of interstitial fibrotic area detected by type I collagen in Mallory–Azan staining, concomitant with the downregulation of transforming growth factor (TGF)-β1 and type I procollagen mRNA expression in damaged kidneys. The CX3CR1 blockade also decreased the number of infiltrating F4/80-positive macrophages in damaged kidneys. These results suggest that the fractalkine-CX3CR1 axis contributes to kidney fibrosis in a hypertensive mouse model, possibly by the upregulation of macrophage infiltration and the expression of TGF-β1 and type I collagen.
Similar content being viewed by others
Introduction
Kidney fibrosis is a characteristic finding of progressive kidney injury that results in organ failure, and it is accepted as the major determinant of the prognosis of kidney diseases. Accumulating evidence suggests that chemokines and their cognate receptors, such as monocyte chemoattractant protein-1/macrophage chemotactic and activating factor/CCL2 and its cognate receptor, CCR2, contribute to chronic kidney inflammation, leading to kidney fibrosis.1, 2, 3, 4 Additionally, secondary lymphatic chemokine/CCL21 and its receptor, CCR7, have a role in kidney fibrosis.5 Recently, fractalkine/CX3CL1, a CX3C chemokine, was found to regulate kidney interstitial fibrosis via its cognate receptor, CX3CR1, after ischemia reperfusion injury in mice.6 To support this notion, patients with systemic sclerosis exhibited high levels of serum fractalkine, concomitantly with the presence of CX3CR1-positive macrophages and T cells in fibrotic skin and lungs.7 Originally, fractalkine/CX3CL1 was reported to be a potent chemoattractant for macrophages, natural killer cells, T cells, mast cells and platelets, and it uniquely serves as an adhesion molecule expressed on endothelial cells.8 Recently, pathophysiological roles of the fractalkine-CX3CR1 axis were noted in the bactericidal host defense during septic peritonitis9 and corneal neovascularization.10 Additionally, fractalkine/CX3CL1 was reported to be involved in the progression of human glomerulopathy, including crescentic glomerulonephritis.11 Collectively, these data suggest that the fractalkine-CX3CR1 axis may promote tissue injury through immune competent cell infiltration and fibrogenesis. However, the impacts of the fractalkine-CX3CR1 axis on progressive interstitial lesions, including kidney fibrosis associated with hypertension, are poorly understood.
Hypertensive kidney disease, including interstitial fibrosis, is often present in patients with long-term hypertension.12 However, the precise underlying mechanisms involved in hypertensive kidney damage remain unclear. Several lines of evidence suggest that the inflammatory processes may have a key role in the pathogenesis of hypertensive kidney damage. Tian et al.13 reported that an increase of oxidant stress in hypertensive kidney disease activated nuclear factor (NF)-κ B, which eventually induced the expression of proinflammatory cytokines and chemokines in the kidney. Moreover, anti-oxidant treatment decreased kidney inflammatory cytokines and chemokines, kidney immune competent cells and NF-κ B, thereby improving kidney function and damage. Thus, the inflammatory process may be a key to interstitial fibrosis in hypertensive kidney.
These findings prompted us to examine whether fractalkine/CX3CL1 and its cognate receptor, CX3CR1, contribute to progressive kidney injury associated with hypertension. In the present study, we tested this hypothesis in a deoxycorticosterone acetate (DOCA)-salt hypertensive model in CX3CR1-deficient mice. In this study, we report that fractalkine-CX3CR1 axis promotes kidney fibrosis with concomitant increases in transforming growth factor (TGF)-β1 and type I collagen in damaged kidneys.
Methods
Animals
Inbred male C57BL/6 mice aged 30 weeks were purchased from Charles River Japan (Yokohama, Japan). The male CX3CR1-deficient mice were on an outbred C57BL/6 genetic background (n>8 generations), and were used at 30 weeks of age.9, 10 Five mice were used in each experiment. All animal experiments were performed at the Institute for Experimental Animals (Kanazawa University Advanced Science Research Center, Kanazawa, Japan), complied with the standards set out in the Guidelines for the Care and Use of Laboratory Animals of Kanazawa University, and were approved by the Committee on Animal Experimentation of Kanazawa University.
Hypertensive kidney injury model
CX3CR1-deficient and wild-type mice were anesthetized with diethyl ether and pentobarbital sodium. A flank incision and left unilateral nephrectomy were made. Then, 21-day-release DOCA pellets containing 50 mg of DOCA (Innovative research of America, Sarasota, FL, USA) were implanted subcutaneously by incision of the right flank. The sham operation was performed in a similar manner, except for unilateral nephrectomy and DOCA implantation for control mice. The DOCA animals received isotonic saline for 28 days starting with the administration of DOCA. Blood pressure was measured using the tail cuff method twice a week until the day of sacrifice. For pathological examination, the remaining right kidney was harvested on day 28.
Tissue preparation
One portion of the kidney tissue was fixed in 10% buffered formalin (pH 7.2), embedded in paraffin, cut at 4 μm, stained with hematoxylin and eosin, periodic acid Schiff's reagent or Mallory–Azan and observed under a light microscope. Two independent observers with no prior knowledge of the experimental design evaluated each section. The mean interstitial fibrotic area, expressed as blue coloration in the Mallory–Azan staining, was evaluated from the whole area of the cortex and outer medulla in the individual complete sagittal kidney section and expressed as a percentage of the field using Mac Scope version 6.02 (Mitani Shoji, Fukui, Japan).
Immunohistochemical studies
The other portion of fresh renal tissue, which was embedded in the OCT compound (Sakura Finetek USA Inc, Torrance, CA, USA) and snap-frozen in n-hexane cooled with a mixture of dry ice and acetone, was cut at 6 μm on a cryostat (Tissue-Tek systems; Miles, Naperville, IL, USA). The presence of F4/80-positive macrophages was detected immunohistochemically using the rat anti-mouse F4/80 monoclonal antibody (clone: A3-1; BMA Biomedicals AG, Augst, Switzerland). The number of interstitial infiltrated F4/80-positive macrophages was counted in the whole area of the cortex and outer medulla where cell migration was maximal, and it was expressed as the mean number±standard error per mm2. The presence of TGF-β1 protein was demonstrated immunohistochemically on formalin-fixed, paraffin-embedded kidney tissue specimens using the indirect avidin-biotinylated peroxidase complex method with rabbit anti-mouse TGF-β1 polyclonal antibodies (clone: sc-146; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The antigen was retrieved with Target Retrieval Solution (DAKO, Glostrup, Denmark). The presence of type I collagen was also demonstrated immunohistochemically on paraffin-embedded kidney tissue with rabbit anti-mouse type I collagen polyclonal antibodies (CHEMICON International, Temecula, CA, USA). The expression of TGF-β1 and type I collagen was evaluated from the whole area of cortex and outer medulla in the individual complete sagittal kidney section, and expressed as a percentage using Mac Scope version 6.02.
The presence of CX3CL1 protein was also demonstrated immunohistochemically on paraffin-embedded kidney tissue with goat anti-rat CX3CL1 polyclonal antibodies (R&D Systems, Minneapolis, MN, USA).9
Detection of type I procollagen, CX3CL1 and CX3CR1 transcripts in kidneys by real-time reverse transcription PCR
To determine the transcripts of type I procollagen, CX3CL1 and CX3CR1, total RNA was extracted from the whole kidneys. The complimentary DNA was reverse transcribed from 1 μg of total RNA using a SuperScript II RNase H− Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed using the following parameters: 10 min at 25 °C, 30 min at 48 °C and 5 min at 95 °C. For all PCR experiments, the Light Cycler (Roche Diagnostics, Basel, Switzerland) was used. Quantitative real-time reverse transcription PCRs were performed on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using 384-well microtiter plates. The reactions were performed in a total volume of 20 μl, containing 1 μl of complimentary DNA sample, TaqMan Gene Expression Assays (Applied Biosystems) and Taqman Universal PCR Master Mix (Applied Biosystems) using the universal temperature cycles: 10 min at 94 °C followed by 40 two-temperature cycles (15 s at 94 °C and 1 min at 60 °C). Assay IDs of TaqMan Gene Expression Assays were Mm00801666 for mouse procollagen type 1, Mm00436454_m1 for mouse CX3CL1, Mm00438354_m1 for mouse CX3CR1 and Mm00446953_m1 for murine β-glucuronidase. The mRNA expression in each sample was finally described after correction with β-glucuronidase expression.
Statistical analysis
The mean number±standard error. were calculated on all parameters determined in this study. Statistical analyses were performed using the analysis of variance test. A value of P<0.05 was accepted as statistically significant.
Results
DOCA mice with unilateral nephrectomy showed the elevation of blood pressure, but a CX3CR1 deficiency hardly affected blood pressure
To determine the impact of the deficiency of CX3CR1 on blood pressure, blood pressure was measured in unilateral nephrectomized mice with or without DOCA administration. Unilateral nephrectomy alone did not increase the blood pressure levels in any mice examined in this study. In contrast, DOCA administration elevated blood pressure to similar extents in both wild-type and CX3CR1-deficient mice (Figure 1).
Deoxycorticosterone acetate (DOCA)-treated uninephrectomized mice developed hypertension. Systolic blood pressure measured by the tail cuff method in the mice of each experimental group. Control, sham-operated control mice; UNx, uninephrectomized mice; DOCA, DOCA-treated uninephrectomized mice; B6, C57BL/6; CX3CR1−/−, CX3CR1-deficient mice. Systolic blood pressure was increased in all mice 14 days and 21 days after DOCA treatment in both B6 and CX3CR1−/− mice. There was no statistical difference in blood pressure between B6 and CX3CR1−/− mice. n=5 in each experiment. Values are expressed as the mean number±standard error.
Fractalkine-CX3CR1 expression in damaged kidneys
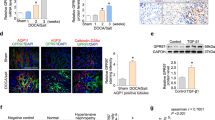
To determine the impact of CX3CR1 deficiency on fractalkine-CX3CR1 expression in the kidney, the presence of transcripts of fractalkine/CX3CL1 and CX3CR1 was evaluated. Fractalkine/CX3CL1 expression was upregulated especially in DOCA-administered mice, which was ameliorated by CX3CR1 deficiency (Figure 2a). The CX3CR1 mRNA expression was upregulated in unilateral nephrectomized mice, which was augmented by DOCA administration (Figure 2b).
Enhanced mRNA expression of CX3CL1 and CX3CR1 in whole kidney by deoxycorticosterone acetate (DOCA) treatment. (a) Transcripts of CX3CL1 were upregulated after DOCA treatment in C57BL/6 mice detected by real-time reverse transcription PCR, which was reduced by CX3CR1 deficiency. (b) Transcripts of CX3CR1 were upregulated after DOCA treatment in C57BL/6 mice detected by real-time reverse transcription PCR. CX3CL1 protein was not detected immunohistochemically in untreated C57BL/6 mice (c) and DOCA-administered CX3CR1−/− mice (d). CX3CL1 was expressed in tubular epithelial cells (e) and vascular endothelial cells (f). n=5 in each experiment. Values are the mean number±standard error. *P<0.05 compared with the control group, #P<0.05 compared with the uninephrectomized group, †P<0.05 compared with CX3CR1-deficient mice. Original magnification is × 200 (c, d and e) and × 400 (f). A full color version of this figure is available at the Hypertension Research journal online.
Furthermore, the fractalkine/CX3CL1 protein was expressed mainly on proximal tubular epithelial cells, peritubular capillaries and vascular endothelial cells in DOCA-administered B6 mice, similar to that reported previously6 (Figures 2e and f). Fractalkine/CX3CL1 protein expression was not upregulated in B6 control mice (Figure 2c) and DOCA-administered CX3CR1−/− mice (Figure 2d).
CX3CR1 deficiency ameliorated kidney interstitial fibrosis
To determine the impact of CX3CR1 on progressive kidney fibrosis, the fibrotic area (indicated in blue on the Mallory–Azan staining) was examined. Untreated mice (Figures 3a and d), sham-operated mice (data not shown) or CX3CR1-deficient mice (Figures 3a and e) exhibited little or no fibrosis. Interstitial fibrosis was observed in damaged kidneys in unilateral nephrectomized mice (Figure 3a). The DOCA treatment further aggravated kidney fibrosis induced by unilateral nephrectomy (Figures 3a and f). Concomitantly, type I collagen protein and mRNA expression levels were upregulated in uninephrectomized mice with DOCA treatment (Figures 3b, c and h). It is important to note that a CX3CR1 deficiency markedly attenuated kidney fibrosis in DOCA-administered mice (Figures 3a and g) and reduced type I collagen in mRNA and protein levels (Figures 3b, c and i).
Deficiency in CX3CR1 reduced kidney interstitial fibrosis. Kidney interstitial fibrosis, expressed as a percentage involvement of the field, was increased in deoxycorticosterone acetate (DOCA)-administered mice (a, f) compared with the DOCA-untreated C57BL/6 mice (a, d) and CX3CR1-deficient mice (a, e). In contrast, the mean interstitial fibrosis was reduced in DOCA-administered CX3CR1-deficient mice (a, g). Type I collagen expression was upregulated in C57BL/6 mice after DOCA treatment (b, h). In contrast, the CX3CR1-deficient mice that received DOCA treatment had a reduced expression of type I collagen (b, i). There was no statistical difference in expression levels between sham-operated C57BL/6 mice and sham-operated CX3CR1-deficient mice (b). Similarly, the mRNA expression of type I procollagen was reduced in CX3CR1-deficient mice compared with that of C57Bl/6 mice (c). n=5 in each experiment. Values are the mean number±standard error. *P<0.05 compared with DOCA-untreated mice, #P<0.05 compared with uninephrectomized group, †P<0.05 compared with CX3CR1-deficient mice. Original magnification is × 400. A full color version of this figure is available at the Hypertension Research journal online.
F4/80-positive macrophages were decreased in number in the CX3CR1-deficient mice
Because fractalkine/CX3CL1 is a potent chemoattractant for macrophages, we examined whether CX3CR1 deficiency impacts interstitial cell infiltration. The F4/80-positive macrophages infiltrated mainly into the outer medulla of damaged kidneys. The number of infiltrating F4/80-positive macrophages in the interstitium of unilateral nephrectomized mice was increased with DOCA administration. On the contrary, CX3CR1 deficiency reduced the number of infiltrated F4/80-positive macrophages in damaged kidneys (Figure 4). Therefore, fractalkine via CX3CR1 affected macrophage infiltration in damaged kidneys in this model.
Infiltration of F4/80-positive cells by deoxycorticosterone acetate (DOCA) treatment was reduced in CX3CR1-deficient mice. F4/80-positive cells were detected using immunofluorescence imaging. The number of F4/80-positive cells was reduced in CX3CR1-deficient mice (a, c) compared with those in wild-type mice (b, c) after DOCA treatment. *P<0.05 compared with the control group, #P<0.05 compared with the uninephrectomized group, †P<0.05 compared with CX3CR1-deficient mice. n=5 in each experiment. A full color version of this figure is available at the Hypertension Research journal online.
Kidney TGF-β1 expression was decreased by CX3CR1 deficiency
To clarify the molecular mechanisms involved in the increase in fibrogenesis caused by CX3CR1 deficiency, the expression of TGF-β1, a potent fibrogenic molecule, was examined. Unilateral nephrectomy enhanced the expression of TGF-β1 protein detected mainly in renal tubular epithelial cells and infiltrating cells in unilateral nephrectomized mice (Figure 5a), compared with normal C57BL/6 mice (Figure 5b) or sham-operated C57BL/6 mice (data not shown). DOCA treatment marginally enhanced TGF-β1 expression induced by unilateral nephrectomy, and it should be noted that CX3CR1 deficiency downregulated TGF-β1 immunoreactivity in damaged kidneys (Figure 5c).
CX3CR1 deletion reduced transforming growth factor (TGF)-β1 expression. (a) TGF-β1 was detected by immunohistochemical staining. Upregulation of TGF-β1 was detected mainly in tubular epithelial cells in C57BL/6 mice after deoxycorticosterone acetate (DOCA) treatment as compared with DOCA-untreated C57BL/6 mice (c). In contrast, the CX3CR1-deficient mice that received DOCA treatment had reduced expression of TGF-β1 (b). (d) TGF-β1 expression was reduced in CX3CR1-deficient mice. n=5 in each experiment. Values are the mean number±standard error. *P<0.05 compared with the control group, †P<0.05 compared with CX3CR1-deficient mice. Original magnification is × 400. A full color version of this figure is available at the Hypertension Research journal online.
Discussion
In the present study, we examined the impact of CX3CR1 signaling on kidney fibrosis in a DOCA-salt hypertensive model using mice that were genetically deficient in CX3CR1. Inhibition of CX3CR1 signaling attenuated progressive kidney fibrosis, concomitant with the downregulation of TGF-β1 and type I procollagen, and a decrease in macrophage infiltration in damaged kidneys. It was also noted that a blockade of CX3CR1 signaling hardly affected the elevation of blood pressure. Taken together, these results suggest that the fractalkine-CX3CR1 axis is required for fibrotic processes involved in progressive hypertensive kidney damage via increasing the expression of type I collagen and TGF-β1 and the infiltration of macrophages.
Fractalkine/CX3CL1 contains a CX3C motif and exists as a membrane-bound glycoprotein with a chemokine domain atop an extended mucin-like stalk.14 Fractalkine/CX3CL1 can be induced on endothelium by inflammatory cytokines, including interleukin-1 and tumor necrosis factor-α. In turn, fractalkine/CX3CL1 has the capacity to be chemotactic to CX3CR1-expressing inflammatory cells, such as macrophages. Macrophage infiltration was observed predominantly in the outer medulla, which is similar to those in other models, such as ischemia-reperfusion,6 kidney fibrosis5 and human kidney disease.15 This result is partly because high endothelial venules (HEVs) that express MECA-79 affect infiltrating cells into kidneys and could be detected in outer medulla;16 however, we did not examine whether HEVs-like vessels in the outer medulla expressed fractalkine/CX3CL1 in this particular model. Recently, fractalkine/CX3CL1 and CX3CR1 have been reported to contribute to fibrogenesis.6 In that report, a CX3CR1 deficiency or blockade attenuated macrophage infiltration and early platelet-derived growth factor-β expression, as well as late-phase interstitial fibrosis and kidney dysfunction after ischemia reperfusion injury. In the present study, the fractalkine-CX3CR1 axis-dependent macrophage infiltration in the damaged kidneys may have a role in the pathogenesis of kidney fibrosis in unilateral nephrectomized mice with DOCA administration. This result might be partly explained by the decrease in TGF-β1 expression in macrophages and/or tubular epithelial cells, which was activated by fractalkine-CX3CR1 pathways.
Endothelial cells are equipped with numerous receptors that allow them to detect and respond to the mechanical forces generated by pressure and shear stress. The cytoskeleton and other structural components have an established role in the mechanotransduction to be able to transmit and modulate tension within the cell via adhesion sites, integrins, cellular junctions and the extracellular matrix. Mechanical forces also initiate complex signal transduction cascades, including the NF-κ B and mitogen-activated protein kinase pathways. Transcription factor NF-κ B in endothelial cells is activated following the exposure to shear stress.17 NF-κ B induces the transcription of a large range of genes implicated in inflammation, including fractalkine/CX3CL1.18 Supporting this notion, dexamethasone suppressed the expression of fractalkine/CX3CL1.19 Alternatively, fractalkine/CX3CL1 has a role in vascular remodeling in pulmonary hypertension.20 A recent report described that fractalkine/CX3CL1 stimulates the phosphorylation of the mitogen-activated protein kinases p38, c-Jun N-terminal kinase and extracellular-regulated kinase 1/2 as well as the serine-threonine kinase Akt at Ser 473 and Thr 308.21 These results may be supportive because mitogen-activated protein kinases have been reported to be involved kidney fibrosis.22 Although this remodeling process remains to be investigated, it would be reasonable to speculate that fractalkine/CX3CL1 induced by hypertensive endothelial cells injury might, in turn, promote vascular remodeling in damaged kidneys. Further study will be required to test this hypothesis.
The expression of fractalkine/CX3CL1 was reduced by the fractalkine-CX3CR1 blockade. This finding may be explained by some speculations. First, this reduction may be dependent on the decrease in the number of macrophages in damaged kidneys. In support of this finding, it has been reported that fractalkine/CX3CL1 is expressed on infiltrating mononuclear cells, such as endothelial cells.23 Second, the interaction of endothelial cells and macrophages may be important. Activated macrophages expressing CX3CR1 might produce proinflammatory cytokines and chemokines, which activate endothelial cells and subsequently promote the inflammatory process. A fractalkine-CX3CR1 blockade may inhibit these processes, leading to anti-inflammatory responses, thereby possibly decreasing the expression of fractalkine/CX3CL1 in kidneys.
In a recent study, it was found that direct mineralocorticoid effects and high blood pressure contributes to fibrosis of the kidney in DOCA-salt hypertension.24 Therefore, in this study, kidney fibrosis might be induced by the mineralocorticoid effect of DOCA and by hypertension. However, DOCA alone did not induce obvious kidney fibrosis in C57BL/6 mice without unilateral nephrectomy in the previously mentioned study.24 Collectively, although the possible direct effect of DOCA should not be neglected, kidney fibrosis in this particular model may be mostly due to systemic hypertension.
In conclusion, the fractalkine-CX3CR1 axis may be involved in the pathogenesis of progressive kidney fibrosis in a hypertensive model, possibly due to the upregulation of TGF-β1 and type I collagen. Thus, blockade of the fractalkine-CX3CR1 axis may be useful in anti-fibrotic therapeutic strategies in progressive kidney fibrosis.
References
Wada T, Matsushima K, Kaneko S . Chemokines and the kidney. In: Linkes WP (ed). Progress in Chemokine Research. Nova Science Publishers: Hauppauge, 2007, pp 179–186.
Wada T, Yokoyama H, Furuichi K, Kobayashi KI, Harada K, Naruto M, Su SB, Akiyama M, Mukaida N, Matsushima K . Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor (MCAF/MCP-1). FASEB J 1996; 10: 1418–1425.
Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H . Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol 2004; 165: 237–246.
Wada T, Furuichi K, Sakai N, Iwata Y, Kitagawa K, Ishida Y, Kondo T, Hashimoto H, Ishiwata Y, Mukaida N, Tomosugi N, Matsushima K, Egashira K, Yokoyama H . Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J Am Soc Nephrol 2004; 15: 940–948.
Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S . Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA 2006; 103: 14098–14103.
Furuichi K, Gao JL, Murphy PM . Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am J Pathol 2006; 169: 372–387.
Hasegawa M, Sato S, Echigo T, Hamaguchi Y, Yasui M, Takehara K . Up-regulated expression of fractalkine/CX3CL1 and CX3CR1 in patients with systemic sclerosis. Ann Rheum Dis 2005; 64: 21–28.
Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O . Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997; 91: 521–530.
Ishida Y, Hayashi T, Goto T, Kimura A, Akimoto S, Mukaida N, Kondo T . Essential involvement of CX3CR1-mediated signals in the bactericidal host defense during septic peritonitis. J Immunol 2008; 181: 4208–4218.
Lu P, Li L, Kuno K, Wu Y, Baba T, Li YY, Zhang X, Mukaida N . Protective roles of fractalkine/CX3CL1-CX3CR1 interactions in alkali-induced corneal neovasculization through enhanced antiangiogenic factor expression. J Immunol 2008; 180: 4283–4291.
Furuichi K, Wada T, Iwata Y, Sakai N, Yoshimoto K, Shimizu M, Kobayashi K, Takasawa K, Kida H, Takeda S, Matsushima K, Yokoyama H . Upregulation of fractalkine in human crescentic glomerulonephritis. Nephron 2001; 87: 312–320.
Kaplan NM . Kaplan's Clinical Hypertension, 9th edn. Lippincott Willams&Wilkins: Philadelphia, 2006, pp 122–137.
Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning Jr RD . Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 2007; 293: H3388–H3395.
Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ . A new class of membrane-bound chemokine with a CX3C motif. Nature 1997; 385: 640–644.
Takaeda M, Yokoyama H, Segawa-Takaeda C, Wada T, Kobayashi K . High endothelialvenule-like vessels in the interstitial lesions of human glomerulonephritis. Am J Nephrol 2002; 22: 48–57.
Segawa C, Wada T, Takaeda M, Furuichi K, Matsuda I, Hisada Y, Ohta S, Takasawa K, Takeda S, Kobayashi K, Yokoyama H . In situ expression and soluble form of P-selectin in human glomerulonephritis. Kidney Int 1997; 52: 1054–1063.
Lan QX, Mercurius KO, Davies PF . Stimulation of transcription factors NFκB and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Communi 1994; 201: 950–956.
Bhabsar OK, Sukkar MB, Khorasani N, Lee KY, Chung KF . Glucocorticoid suppression of CX3CL1(fractalkine) by reduced gene promoter recruitment of NF-kappa B. FASEB J 2008; 22: 1807–1816.
Yoshida T, Ishikawa I, Omo Y, Imai T, Suzuki R, Yoshie O . An activation-responsive element in single C motif-1/lymohotactin promoter is a site of constitutive and inducible DNA-protein interactions involving nuclear factor of activated T cell. J Immunol 1999; 163: 3295–3303.
Schober A, Zernecke A . Chemokines in vascular remodeling. Thromb Haemost 2007; 97: 730–737.
Klosowska K, Volin MV, Huynh N, Chong KK, Halloran MM, Woods JM . Fractalkine functions as a chemoattractant for osteoarthritis synovial fibroblasts and stimulates phosphorylation of mitogen-activated protein kinases and Akt. Clin Exp Immunol 2009; 156: 312–319.
Wada T, Furuichi K, Sakai N, Hisada Y, Kobayashi K, Mukaida N, Tomosugi N, Matsushima K, Yokoyama H . Involvement of p38 mitogen-activated protein kinase followed by chemokine expression in crescentic glomerulonephritis. Am J Kidney Dis 2001; 38: 1169–1177.
Suzuki F, Nanki T, Imai T, Kikuchi H, Hirohata S, Kohsaka H, Miyasaka N . Inhibition of CX3CL1(fractalkine) improves experimental autoimmune myositis in SJL/J mice. J immunol 2005; 175: 6987–6996.
Klanke B, Cordasic N, Hartner A, Schmieder RE, Veelken R, Hilgers KF . Blood pressure versus mineralocorticoid effects on kidney inflammation and fibrosis in DOCA-salt hypertension. Nephrol Dial Transplant 2008; 23: 3456–3463.
Acknowledgements
This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan. There is no conflict of interest in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimizu, K., Furuichi, K., Sakai, N. et al. Fractalkine and its receptor, CX3CR1, promote hypertensive interstitial fibrosis in the kidney. Hypertens Res 34, 747–752 (2011). https://doi.org/10.1038/hr.2011.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.23
Keywords
This article is cited by
-
Regulation and function of CX3CR1 and its ligand CX3CL1 in kidney disease
Cell and Tissue Research (2021)
-
Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration
Scientific Reports (2017)
-
Role of CX3CL1 in Diseases
Archivum Immunologiae et Therapiae Experimentalis (2016)
-
Methylprednisolone attenuates lipopolysaccharide-induced Fractalkine expression in kidney of Lupus-prone MRL/lpr mice through the NF-kappaB pathway
BMC Nephrology (2015)
-
Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1
Stem Cell Research & Therapy (2014)