Abstract
Twelve populations of the epiphytic bryophyte Leucodon sciuroides from three major regions representing formerly glaciated and nonglaciated regions of Europe were screened for polymorphisms at 15 putative isozyme loci. The populations clustered into three distinct groups consisting of: (i) a single population from Crete, representing a cryptic unknown taxon; (ii) four Scandinavian populations and two populations from northern Greece; and (iii) the remaining populations from mainland Greece and Crete. The Scandinavian populations were genetically depleted compared with most Greek populations, thus fitting the expectation of generally lower levels of variation in formerly glaciated areas. The transition zone between genetically diverse and depleted populations appears to be located through northern Greece, coinciding with the northern limit of the Mediterranean region. This indicates that genetic variation was lost in populations at the northern limit of glacial refugia. The two groups of populations fit a progenitor–derivative model. They also have contrasting reproductive strategies: the Mediterranean populations reproduce sexually, whereas the other populations propagate vegetatively. Epiphytic species, growing on substrates that are limited in space and time, appear to be especially vulnerable to loss of genetic variation. Lack of genetic variation and therefore low adaptability to increased levels of atmospheric pollution may explain why many epiphytic lichen and bryophytes, including L. sciuroides, are declining over much of Europe.
Similar content being viewed by others
Introduction
Populations of plants and animals are generally believed to have reduced levels of intraspecific variation in areas severely affected by the Pleistocene glaciations (Taberlet et al., 1998). Genetic variation is expected to have been lost through bottleneck effects during survival in geographically restricted refugia and through repeated founder events during recolonization (Hewitt, 1996). Few plant species, mostly trees, have been screened for genetic variation on a broad geographical scale in Europe. Among those, few show clearly the expected distribution of variation. For example, data from six isozyme loci in beech (Fagus sylvatica L.) do not show any general trend with respect to gene diversity between Mediterranean and continental regions of Europe (Comps et al., 1990). In contrast, the European silver fir (Abies alba Mill.) is genetically more variable in the Mediterranean region than in central and north-eastern Europe (Bergmann et al., 1990), but increased levels of variation exist where remigrated populations from different refugia meet (Konnert & Bergmann, 1995). Central European populations of Norwegian spruce (Picea abies L.) are genetically depauperate, probably as an effect of severe restrictions of population size at glacial refugia in the Dinaric Alps and in the Carpathians, whereas populations that are believed to have colonized Scandinavia from a refugium in the present-day area of Moscow have retained more variation (Lagercrantz & Ryman, 1990). On a slightly narrower geographical scale exist several examples of herbs with reduced levels of variation towards the north (e.g. Calluna vulgaris L. Hull (Mahy et al., 1997) and Carex arenaria L. (Jonsson, 1998)). The Mediterranean region, especially the eastern Mediterranean region, is poorly represented in studies of genetic diversity (Affre & Thompson, 1997; Thompson, 1999).
To evaluate the genetic effects of Pleistocene glaciations, there is a need to study plants that represent a greater diversity of taxonomic groups with contrasting breeding systems and ecological demands (cf. Vogel et al., 1999). Since bryophytes have wide distribution areas that extend over several climatic zones, they are well suited for testing hypotheses on regional infraspecific variability and differentiation. Another important aspect is the genetic consequences of the haploid status of the dominant phase of the bryophyte life cycle (Wyatt, 1982).
To test the hypothesis that populations in formerly glaciated areas are genetically depleted, I compared isozyme diversity of some widespread bryophyte species. Within this project, populations originating from Scandinavia and Greece were chosen to exemplify formerly glaciated and less affected regions, respectively. Although Greek populations must also have experienced drastic climatic changes during the glacial cycles, they could have moved relatively short distances up and down the mountain slopes following the climatic oscillations (Hewitt, 1996). This study of Leucodon sciuroides (Hedw.) Schwaegr. was included in the larger project to represent an epiphytic life form with a unisexual breeding system and dispersal via both asexual and sexual reproduction.
Materials and methods
The plant
Leucodon sciuroides is widespread in the Palaearctic. In Europe it reaches north to Iceland and northern Scandinavia (Pippo, 1982; Nyholm, 1954–60) and south to the Mediterranean region, where it is a dominant epiphyte above the coastal plains (Preston, 1994). In Scandinavia L. sciuroides usually grows as an epiphyte on trunks of lowland deciduous trees with rich bark, either in forests or along roadsides. To the north, it occurs occasionally up to the subalpine region on basic rock (Mårtensson, 1956; Pippo, 1982). In Greece, it grows on trunks of a variety of trees, usually in mountain areas with comparatively high levels of precipitation. The size is variable, and more robust plants, which are common in the Mediterranean area, are sometimes recognized as var. morensis (Limpr.) De Not. The shoots are unisexual and the spores are 24–28 μm in diameter (Nyholm, 1954–60), but sporophytes are, according to records (Möller, 1912; Preston, 1994; Nyholm, 1954–60), rarely observed in populations from northern Europe. Instead, vegetative reproduction takes place by dispersal of specialized brood branches. Because of atmospheric pollution, L. sciuroides is declining in southern Scandinavia (Sjögren, 1995) and in Great Britain (Preston, 1994; Bates et al., 1997), as well as in central and western Europe (Preston, 1994; Koperski, 1998).
Sampling procedure and sites
Four populations of L. sciuroides were collected from each of three major regions: (i) Scandinavia; (ii) mainland Greece; and (iii) the island of Crete in the Greek part of the Aegean Sea (Table 1; Fig. 1).
Geographical location of investigated populations of Leucodon sciuroides in Scandinavia and Greece. Abbreviations for populations are given in Table 1.
Plant material was collected from 10 to 15 trees at each site (at AM from a single vertical cliff). As the local populations were quite unevenly distributed among the tree trunks, I chose to make the sampling effort proportional to the coverage at each particular stem. Each sample came from a separate colony along the trunk, or was separated by 40 cm or more from other samples if the coverage was continuous. The number of samples gathered from each stem therefore varied from one to eight. In total, ≈40 samples were taken from each site. Some populations were small, so that fewer samples had to be collected.
Each sample was divided into two subsamples: one was kept as a voucher specimen; the other was cultured until extraction. One or two specimens from each tree were examined for presence of antheridia or archegonia, sporophytes, and brood branches.
Electrophoretic procedures
The top centimetre of lateral branches was extracted following procedures described by Cronberg (1995) [buffer B]. The gel–electrode buffer systems used were (i) a lithium–borate buffer (Soltis et al., 1983; no. 7), which ran at 50 mA for 4 h and (ii) a histidine–citrate buffer (Wendel & Weeden, 1989; no. 1; adjusted to pH 6.5), which ran at 30 mA for 3 h. Electrophoresis was performed on 6 mm thick, horizontal 10.5% starch gels. After separation, enzymes were stained using standard colorimetric methods (Wendel & Weeden, 1989). The lithium–borate buffer resolved aspartate aminotransferase (AAT, EC 2.6.1.1), glucose-6-phosphate isomerase (GPI, EC 5.3.1.9), triosephosphate isomerase (TPI, EC 5.3.1.1), fructose-bisphosphate aldolase (FBA, EC 4.1.2.13), and superoxide dismutase (SOD, EC 1.15.1.1). The resolution of banding patterns was significantly improved when this electrode buffer was recycled once. The histidine–citrate buffer resolved aconitase (ACO, EC 4.2.1.3) isocitrate dehydrogenase (IDH, EC 1.1.1.42), malate dehydrogenase (MDH, EC 1.1.1.37), phosphoglucomutase (PGM, EC 5.4.2.2), and UDP pyrophosphorylase (UGPP; EC 2.7.7.9; Manchenko, 1994).
Homogenate from a single clone of the moss Pleurozium schreberi (Brid.) Mitt. was placed at five positions on every gel to provide a control against which the relative mobility of the bands in the other individuals could be scored. Enzyme loci were numbered in relation to their migration distance, beginning at the anodal end of the gel, with alleles ordered alphabetically, the a-allele of each locus having the longest migration distance.
Data analysis
Gene frequencies were analysed using B I O S Y S-1 release 1.7 (Swofford & Selander, 1981). Mean number of alleles per locus (A), percentage polymorphic loci (P), and allelic diversity (HS, unbiased estimate; Nei, 1975, 1978) were used as measures of genetic variability within populations. Cavalli-Sforza & Edwards’s (1967) pairwise chord distances between populations were summarized into a UPGMA phenogram. The FSTAT software (Goudet, 1995) was used to calculate Weir & Cockerham’s (1984) estimate of FST (theta). Means and variances were obtained by jackknifing over loci. All analyses were performed at ramet level.
At tree trunk level, the numbers of detected genotypes were compared with the numbers of samples in order to check whether the populations at individual trees consisted of one or more genotypes.
Results
Reproduction
The Scandinavian plants reproduced exclusively by means of vegetative brood branches. Brood branches were abundant also in the RO and VO populations, but scarce in the OL population and lacking in the remaining populations. Sexual reproduction showed a reverse trend, such that the populations from Crete, with the exception of the KA population, had colonies with sporophytes. Occasional sporophytes were found also in OL and RO. No antheridia or archegonia could be detected and no sporophytes were found in the Scandinavian populations (Table 2).
Interpretation of banding patterns
All enzymes screened by electrophoresis could be scored as discrete loci with the expected haploid expression. Of 15 putative enzyme loci, only two (Fba-1, Sod-1) were monomorphic. AAT showed activity at three loci, two of which were possible to score (Aat-1, Aat-2). GPI showed activity at two loci, one of which (Gpi-2) could be used. TPI had two loci. In ACO two close zones of activity were revealed, which were interpreted as two separate loci (Aco-1 and Aco-2), although activity at one of these (Aco-1) was sometimes lacking. It seems probable that a duplication has taken place in the past, and subsequently some alleles at Aco-1 may have lost their activity without lethal effect. The same phenomenon appears to occur in PGM, which also exhibited two close zones of bands (Pgm-2 and Pgm-3). A third, faster migrating zone of bands (Pgm-1) was too diffuse to allow screening. For IDH a single zone of activity was detected (Idh-1), expressed as double or triple bands, which were interpreted as post-translational modifications. Similarly, MDH showed triple bands at one zone of activity (Mdh-1). UGPP exhibited one locus with two alleles.
Variability within populations
Scandinavian populations had low variability within populations (Table 2), exhibiting consistently low numbers of alleles per locus (A=1.1–1.3), low percentages of polymorphic loci (P=7.2–28.6) and low allelic diversity (HS=0.036–0.084), as well as low numbers of detected genotypes (G=2–4). The lowest overall values were found in the population at the northern distributional limit (AM). Except for the RO population, genetic variability was much higher in the Greek populations. This pattern was consistent at almost all polymorphic loci, and with all measures of genetic variability.
Differentiation between populations and regions
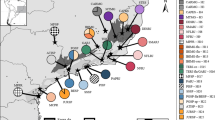
A comparison of pairwise genetic distances between populations showed three groups of populations (Fig. 2). The Scandinavian populations group together with the two populations from northern Greece, whereas the remaining populations from mainland Greece and three populations from Crete group together in a second major group. The allelic pool of the former group represents a subset of the most common alleles of the latter group. One of the populations from Crete (KA) is apparently unrelated to any of the other populations, representing an unidentified taxon. It was collected in a lowland area that is known for disjunct populations of tropical ferns.
UPGMA phenogram expressing overall levels of genetic similarity among 12 populations of Leucodon sciuroides. The phenogram is based on Cavalli-Sforza & Edwards’s (1967) chord distance using frequencies from 15 putative isozyme loci scored at the ramet level.
If the KA population is excluded, the relative differentiation among the remaining populations is fairly high; FST=0.437 ± 0.102. When these populations are separated into the two major groups, the relative differentiation within each group is considerably lower; 0.116 ± 0.053 for the Scandinavian and two northern Greek populations; and 0.088 ± 0.018 for the three Crete, OL, and PA populations. The relative differentiation within the subgroup consisting of the three Scandinavian populations (GY, VI, HB) and one of the northern Greece populations (RO) is even lower (0.049 ± 0.065).
Within-tree variability
In the Greek populations, most tree trunks with L. sciuroides were populated by numerous clones, which could be accurately identified by their haplotypes because of high levels of isozyme variability. Figure 3(a) illustrates the relationship between numbers of sampled colonies and numbers of detected haplotypes from three sexually reproducing populations on Crete. Multiple clones, although to a lesser extent, occur also on trees in Scandinavian populations (Fig. 3b). The cliff population at AM, northern Sweden, had the lowest number of haplotypes: only two.
Scatter plot illustrating the relationship between number of colonies of Leucodon sciuroides sampled from each tree trunk and number of haplotypes detected by isozyme electrophoresis. Trees from which only one colony was sampled have been omitted. (a) Data compiled from three populations from the island of Crete (DI, OM and XI; N=23 trees). (b) Data compiled from three Scandinavian populations (GY, HB and VI; N=22 trees).
Discussion
Genetic diversity
Populations of L. sciuroides from formerly glaciated Scandinavian areas and the two Greek populations from the northern limit of the Mediterranean region (RO and VO) are clearly genetically depleted compared with the group of populations consisting of OL and PA from the Greek mainland and the populations from Crete. The genetic differentiation within this latter group of populations (FST=0.088; the divergent KA population excluded) was low despite the fact that Crete has been isolated from the mainland for several millions of years and has a correspondingly distinctive flora. This indicates that gene flow, probably by spores, across the Aegean Sea is sufficient to prevent substantial genetic drift. No comparable study of genetic differentiation across the Aegean Sea seems to exist, but Affre & Thompson (1997) found a considerably higher level of differentiation (FST=0.168) among seven populations of Cyclamen creticum Hildebr. from Crete.
The Mediterranean populations of L. sciuroides have similar levels of variation to those reported for several species of Leucodon from Japan (Akiyama, 1994). Wyatt et al. (1993) have suggested that events related to the Pleistocene glaciations are possible explanations for low levels of variation in the European endemic mosses Plagiomnium elatum (Bruch & Schimp.) T. Kop. and P. affine (Bland) T. Kop. as compared with related North American species. Their data also indicated that several species distributed on both continents were more variable in North America. Similarly, low levels of genetic variation in a number of liverworts have been interpreted as a consequence of glaciation (Wyatt et al., 1993). However, regarding one of these, P. porelloides (Nees) Lindenb., Cronberg (2000) has shown that Greek populations are as genetically depleted as populations from Poland and Scandinavia. In contrast, the boreal moss Hylocomium splendens (Hedw.) Bruch & Schimp. appears to have its European centre of variation in northern Scandinavia (Cronberg et al., 1997; N. Cronberg, unpubl.), indicating that populations of Scandinavian bryophytes are not necessarily genetically depleted. The most probable reason for this heterogeneity is that H. splendens is more resistant to low temperatures and therefore may have survived in refugia further to the north.
Postglacial recolonization
Leucodon sciuroides is primarily an epiphyte; hence, it probably recolonized formerly glaciated areas following establishment of a cover of suitable host trees. Pollen diagrams show that, for example, Ulmus reached northern Scandinavia during the mid-Holocene temperature maximum at ≈6000 BP (Huntley, 1988), although recent observations of Ulmus glabra Huds. macrofossils suggest that the initial colonization was much earlier, at ≈8500 BP (Kullman, 1998). According to an alternative possible scenario for recolonization, L. sciuroides first established by long-range dispersal to areas with suitable rock and then secondarily colonized tree trunks when the trees eventually arrived. Mårtensson (1956) remarked that the northern populations grow on boulders and rocks that are used as bird perches, suggesting dissemination by birds. Under the first scenario, colonization would have proceeded gradually, which would allow more alleles to persist, resulting in less genetic divergence among populations and areas (Hewitt, 1996). Under the second scenario, it would be expected that the few initial dispersants, representing a biased sample of the total gene pool, would expand rapidly, giving rise to populations with low levels of variation (Hewitt, 1996). Diaspores of bryophytes are haploid, which is also likely to contribute to the depletion of genetic variability under these conditions. Later migrants would contribute little to the gene pool, because they would be entering established populations.
Although the Scandinavian populations have low levels of variation, they are quite similar to the RO population, and to a slightly lesser extent, to the VO population. Both areas represented by RO and VO have a large number of boreal elements, such as Vaccininium myrtillus L. and Picea abies. The Balkan area has been proposed as a major glacial refugium for a number of organisms (Huntley, 1988; Bennett et al., 1991; Taberlet et al., 1998; Hewitt, 1999). The Scandinavian populations could well be derived from the same refugium as the RO population, without loss of alleles. Therefore a gradual, slow expansion seems more likely than a fast expansion. Also, the reduction of genetic variation is likely to be a consequence of processes acting in the glacial refugium, rather than processes acting during recolonization. Being fairly well adapted to low temperatures, L. sciuroides should have been able to survive the last glaciation further to the north than northern Greece, but it is possible that access to suitable host trees has been limiting. It is still possible that the morphologically divergent northern populations growing on rock (Mårtensson, 1956), here represented by the nearly monomorphic AM population, have reached their outposts by long-range dispersal.
Sexual vs. vegetative reproduction
All populations from Scandinavia and the RO and VO populations from northern Greece, differ from the Mediterranean populations by predominantly vegetative propagation and a virtual absence of sexual reproduction. An intermediate position in this respect is represented by the OL population, which combines presence of both vegetative and sexual reproduction with a high level of variation. Epiphytic bryophytes are likely to be predisposed for efficient dispersal, because their habitat is discontinuous both in time and space. According to During (1979), L. sciuroides and many other epiphytes should be referred to as perennial shuttle species, typically with a comparatively long life-span, moderate sexual reproductive effort, large spores, and frequent production of asexual propagules. Dispersal by brood branches is likely to be more efficient than sexual reproduction during a colonization phase, because the time required from establishment on a tree trunk to production of the next generation of brood branches is much shorter than the time required to complete the sexual cycle. Furthermore, for sexual reproduction to take place, it is necessary that both sexes have arrived within fertilization distance, which in bryophytes is restricted to few centimetres (Wyatt, 1982). Ferns of the genus Asplenium have similar reproductive constraints (Vogel et al., 1999). In obligately outbreeding diploid populations, different plants must grow in close proximity for fertilization to take place, but polyploid derivatives have the capacity for intragametophytic selfing and therefore the potential to establish fertile populations from single spores. According to Vogel et al. (1999), diploid populations of Asplenium are confined to glacial relict areas around the Mediterranean basin, whereas polyploid derivatives, with greater dispersal potential, have dispersed to Scandinavia and the British Isles.
The dominance of vegetative reproduction could alternatively be explained by spore dispersal during the mid-Holocene temperature maximum, leaving the re-migrated populations dependent on vegetative reproduction as the climate deteriorated.
The first suggestion seems more likely because all populations with frequent production of brood branches are apparently more closely related to each other than to the remaining, largely sexually reproducing populations. The two groups of populations also fit a progenitor–derivative model (Crawford, 1983), in which the gene pool of the derivative is a subset of the gene pool of the progenitor. The shoots are smaller, indicating that production of brood branches is connected with retention of a juvenile phase and perhaps prevention of the development of sexual structures. A trade-off between production of vegetative propagules in early development and production of sexual structures in maturing shoots seems to be common in bryophytes (Schofield, 1981; Mishler, 1988). Several similar cases of species pairs with sexually and vegetatively reproducing taxa occur among Asian Leucodon species (H. Akiyama, pers. comm.).
Within-tree variation
The ability to identify clones depends on the number of variable loci in a population. Thus, it is questionable whether the low number of observed haplotypes per tree in Scandinavian populations is caused by the actual existence of few clones or, rather, by low power of resolution of isozyme markers. The documented predominance of vegetative reproduction, however, suggests that populations consist of few, widely spread clones. The high number of clones per tree in the Mediterranean region indicates that the tree stands act as a metapopulation system (Söderström, 1998), with frequent dispersal between trees so that the system is habitat-limited rather than dispersal-limited.
Genetic diversity and environmental change
The loss of genetic diversity is expected to reduce the ability of a population to respond to environmental change (Falconer, 1989) and to increase the likelihood of its extinction (e.g. Lande & Barrowclough, 1987; Hoffmann & Blows, 1993). This idea has not been verified by empirical studies, but it has been possible to confirm reduced levels of both morphological variance (Whitlock & Fowler, 1999) and allozyme diversity (Leberg, 1992) in experimentally inbred populations. Bergmann et al. (1990) suggested a link between the decline in population size of European silver fir (Abies alba) in Central Europe and low potential for physiological and evolutionary adaptation. During recent decades a large number of epiphytes, including both mosses and lichens, have become increasingly rare primarily because of detrimental effects of atmospheric pollution (Hallingbäck, 1992; Sjögren, 1995). Low levels of variation have been observed in several epiphytic bryophytes that are currently declining in North and Central Europe, like L. sciuroides (this investigation), Neckera pennata (Appelgren & Cronberg, 1999) and Porella platyphylla (L.) Pfeiff. (Boisselier-Dubayle et al., 1998). It is likely that populations of epiphytes, and other ecological groups of bryophytes with relatively short turn-over time of habitats, are more often exposed to genetic depletion through population bottlenecks than species that live in more stable habitats. Genetically diverse populations are more likely to contain alleles that are beneficial in a changing environment, which, because the haploid genomes are directly selected, could rapidly penetrate the populations, either by sexual or by vegetative reproduction. Because of their exposed habitat, epiphytes are likely to be vulnerable to atmospheric pollution, but it is also probable that low levels of genetic variation reduce the potential for genetic adaptation to this form of environmental change.
References
Affre, L. and Thompson, J. D. (1997). Population genetic structure and levels of inbreeding depression in the Mediterranean island endemic Cyclamen creticum (Primulaceae). Biol J Linn Soc, 60: 527–549.
Akiyama, H. (1994). Allozyme variability within and among populations of the epiphytic moss Leucodon (Leucodontaceae: Musci). Am J Bot, 81: 1280–1287.
Appelgren, L. and Cronberg, N. (1999). Genetic and morphological variation in the rare epiphytic moss Neckera pennata Hedw. J Bryol, 21: 97–107.
Bates, J. W., Proctor, M. C. F., Preston, C. D., Hodgetts, N. G. and Perry, A. R. (1997). Occurrence of epiphytic bryophytes in a ‘tetrad’ transect across southern Britain. 1. Geographical trends in abundance and evidence of recent change. J Bryol, 19: 685–714.
Bennett, K. D., Tzedakis, P. C. and Willis, K. J. (1991). Quaternary refugia of north European trees. J Biogeogr, 18: 103–115.
Bergmann, F., Gregorius, H. -R. and Larsen, J. B. (1990). Levels of genetic variation in European silver fir (Abies alba) Are they related to the ‘species’ decline?. Genetica, 82: 1–10.
Boisselier-Dubayle, M. C., Lambourdiere, J. and Bishler, H. (1998). The leafy liverwort Porella baueri (Porellaceae) is an allopolyploid. Pl Syst Evol, 210: 175–197.
Cavalli-Sforza, L. L. and Edwards, A. W. F. (1967). Phylogenetic analysis: models and estimation procedures. Evolution, 21: 550–570.
Comps, B., Thiébaut, B., Paule, L., Merzeau, D. and Letouzey, J. (1990). Allozymic variability in beechwoods (Fagus sylvatica L.) over central Europe: spatial differentiation among and within populations. Heredity, 65: 407–417.
Crawford, D. J. (1983). Phylogenetic and systematic inferences from electrophoretic studies. In: Tanksley, S. D. and Orton, T. J. (eds) Isozymes in Plant Genetics and Breeding, Part A, pp. 257–287. Elsevier, Amsterdam.
Cronberg, N. (1995). Isozyme electrophoresis of Sphagnum an analysis of methodology. Lindbergia, 20: 40–48.
Cronberg, N. (2000). Absence of genetic variation in populations of the liverwort Plagiochila porelloides from northern Greece and southern Scandinavia. Lindbergia, 25: 20–24.
Cronberg, N., Molau, U. and Sonesson, M. (1997). Genetic variation in the clonal bryophyte Hylocomium splendens at hierarchical geographical scales in Scandinavia. Heredity, 78: 239–301.
During, H. J. (1979). Life strategies of bryophytes, a preliminary review. Lindbergia, 5: 2–18.
Falconer, D. S. (1989). Introduction to Quantitative Genetics, 3rd edn. John Wiley and Sons, New York.
Goudet, J. (1995). FSTAT, version 1.2, a computer program to calculate F-statistics. J Hered, 86: 485–486.
Hallingbäck, T. (1992). The effect of air pollution on mosses in southern Sweden. Biol Conserv, 59: 163–170.
Hewitt, G. M. (1996). Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc, 58: 247–276.
Hewitt, G. M. (1999). Post-glacial re-colonization of European biota. Biol J Linn Soc, 68: 87–112.
Hoffmann, A. A. and Blows, M. W. (1993). Evolutionary genetics and climate change, will animals adapt to global warming?. In: Kareiva, P. M., Kingsolver, J. G. and Huey, R. B. (eds) Biotic Interactions and Global Change, pp. 165–178. Sinauer, Sunderland, MA.
Huntley, B. (1988). Europe. In: Huntley, B. and Webb, T., III (eds) Vegetation History, pp. 341–383. Kluwer Academic Publishers, Dordrecht.
Jonsson, B. O. (1998). Genetic Variation, Clonal Diversity and Breeding Systems in Sedges (Carex). Thesis, Lund University, Lund.
Konnert, M. and Bergmann, F. (1995). The geographical distribution of genetic variation of silver fir (Abies alba, Pinaceae) in relation to its migration history. Pl Syst Evol, 196: 19–30.
Koperski, M. (1998). Distribution and phytosociology of slightly acidophilic to slightly basophilic epiphytic bryophytes in old stands of oaks and beeches in the lowlands of Lower Saxony (NW-Germany). Herzogia, 13: 63–80.
Kullman, L. (1998). Non-analogous tree flora in the Scandes Mountains, Sweden, during the early Holocene – macrofossil evidence of rapid geographic spread and response to palaeoclimate. Boreas, 27: 153–161.
Lagercrantz, U. and Ryman, N. (1990). Genetic structure of Norway spruce (Picea abies), concordance of morphological and allozymic variation. Evolution, 44: 38–53.
Lande, R. and Barrowclough, G. F. (1987). Effective population size, genetic variation, and their use in population management. In: Soulé, M. E. (ed.) Viable Populations for Conservation, pp. 87–124. Cambridge University Press, Cambridge.
Leberg, P. L. (1992). Effects of population bottlenecks on genetic diversity as measured by allozyme electrophoresis. Evolution, 46: 477–494.
Mahy, G., Vekemans, X., Jacquemart, A. L. and de Sloover, J. R. (1997). Allozyme diversity and genetic structure in south-western populations of heather, Calluna vulgaris. New Phytol, 137: 325–334.
Manchenko, G. P. (1994). Handbook of Detection of Enzymes on Electrophoretic Gels. CRC Press, Boca Raton, FL.
Mårtensson, O. (1956). Bryophytes of the Torneträsk area, northern Swedish Lapland. II. Musci. K Sv Vetenskapsakad Avh Naturskydd, 14: 1–321.
Mishler, B. D. (1988). Reproductive ecology of bryophytes. In: Lovett Doust, J. and Lovett Doust, L. (eds) Plant Reproductive Ecology: Patterns and Strategies, pp. 284–306. Oxford University Press, New York.
Möller, H. (1912). Löfmossornas utbredning i Sverige. II. Cryphaceae och Neckeraceae. Arkiv för Botanik, 12 (4), 1–83.
Nei, M. (1975). Molecular Population Genetics and Evolution. North Holland, Amsterdam.
Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89: 583–590.
Nyholm, E. (1954–60). Moss Flora of Fennoscandia II. Musci. C. W. K. Gleerups, Lund.
Pippo, S. (1982). Epiphytic bryophytes as climatic indicators in eastern Fennoscandia. Acta Bot Fenn, 119: 1–39.
Preston, C. D. (1994). Leucodon sciuroides. In: Hill, M. O., Preston, C. D. and Smith, A. J. E. (eds) Atlas of the Bryophytes of Britain and Ireland, vol. 3, Mosses (Diplolepidae), p. 214. Harley Books, Colchester.
Schofield, W. B. (1981). Ecological significance of morphological characters in the moss gametophyte. Bryologist, 84: 149–165.
Sjögren, E. (1995). Changes in the Epilithic and Epiphytic Moss Cover in Two Deciduous Forest Areas on the Island of Öland (Sweden) – a Comparison Between 1958 and 1962 and 1988–90. Studies in plant ecology, vol. 19. Svenska Växtgeografiska Sällskapet, Uppsala.
Söderström, L. (1998). Modelling the dynamics of bryophyte populations. In: Bates, J. W., Ashton, N. W. and Duckett, J. G. (eds) Bryology for the Twenty-first Century, pp. 321–330. The Centenary Symposium of the British Bryological Society. Maney Publishing, Leeds.
Soltis, D. E., Haufler, C. H., Darrow, D. C. and Gastony, G. J. (1983). Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedules. Am Fern J, 73: 9–27.
Swofford, D. L. and Selander, R. B. (1981). B I O S Y S -1. A computer program for the analysis of allelic variation in population genetics and biochemical systematics. Release 1.7. University of Illinois, Urbana, IL.
Taberlet, P., Fumagalli, L., Wust-Saucy, A. -G. and Cosson, J. -F. (1998). Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol, 7: 453–464.
Thompson, J. D. (1999). Population differentiation in Mediterranean plants: insights into colonization history and the evolution and conservation of endemic species. Heredity, 82: 229–236.
Vogel, J. C., Rumsey, F. J., Schneller, J., Barett, J. A. and Gibby, M. (1999). Where are the glacial refugia in Europe? Evidence from pteridophytes. Biol J Linn Soc, 66: 23–37.
Weir, B. S. and Cockerham, C. C. (1984). Estimating F-statistics for the analysis of population structure. Evolution, 38: 1358–1370.
Wendel, J. F. and Weeden, N. F. (1989). Visualization and interpretation of plant isozymes. In: Soltis, D. E. and Soltis, P. S. (eds) Isozymes in Plant Biology, pp. 5–45. Dioscorides Press, Portland, OR.
Whitlock, M. C. and Fowler, K. (1999). The changes in genetic and environmental variance with inbreeding Drosophila melanogaster. Genetics, 152: 345–353.
Wyatt, R. (1982). Population ecology of bryophytes. J Hattori Bot Lab, 52: 179–198.
Wyatt, R., Odrzykoski, I. J. and Stoneburner, A. (1993). Isozyme evidence regarding the origins of the allopolyploid moss Plagiomnium curvatulum. Lindbergia, 18: 49–58.
Acknowledgements
I thank Theophanis Constantinidis for serving as an excellent guide in the Greek mountains. Arne Strid provided working space and logistical support. Hiroyuki Akiyama, Mikael Hedrén, and John H. Thompson offered helpful comments on a previous version of the manuscript. This work was initiated during a postdoctoral stay at the Botanical Laboratory, Copenhagen University, sponsored by an EU HCM grant, and completed with support from the Swedish Council for Forestry and Agricultural Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cronberg, N. Genetic diversity of the epiphytic bryophyte Leucodon sciuroides in formerly glaciated versus nonglaciated parts of Europe. Heredity 84, 710–720 (2000). https://doi.org/10.1046/j.1365-2540.2000.00719.x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1046/j.1365-2540.2000.00719.x
Keywords
This article is cited by
-
Plant phylogeography of the Balkan Peninsula: spatiotemporal patterns and processes
Plant Systematics and Evolution (2022)
-
Postglacial-period and Recent Invasions Shape the Population Genetics of Botryllid Ascidians along European Atlantic Coasts
Ecosystems (2006)
-
Ubiquitous genetic diversity in ISSR markers between and within populations of the asexually producing moss Pleurochaete squarrosa
Plant Ecology (2006)