Abstract
Southern Italy has a long history of human occupation and passage of different cultures since the Early Holocene. Repeated, ancient introductions of pigs in several geographic areas in Europe make it difficult to understand pig translocation and domestication in Italy. The archeozoological record may provide fundamental information on this, hence shedding light on peopling and on trading among different ancient cultures in the Mediterranean. Yet, because of the scanty nature of the fossil record, ancient remains from human-associated animals are somewhat rare. Fortunately, ancient DNA analysis as applied to domestic species proved to be a powerful tool in revealing human migrations. Herein, we analyzed 80-bp fragment of mitochondrial DNA control region from 27 Sus scrofa ancient samples retrieved from Southern Italian and Sardinian archeological sites, spanning in age from the Mesolithic to the Roman period. Our results surprisingly indicate the presence of the Near Eastern haplotype Y1 on both Italy’s major islands (Sardinia and Sicily) during the Bronze Age, suggesting the seaborne transportation of domestic pigs by humans at least during 1600–1300 BC. The presence of the Italian E2 clade in domestic contexts shows that the indigenous wild boar was effectively domesticated or incorporated into domestic stocks in Southern Italy during the Bronze Age, although the E2 haplotype has never been found in modern domestic breeds. Pigs belonging to the endemic E2 clade were thus traded between the Peninsula and Sardinia by the end of the second millennium BC and this genetic signature is still detected in Sardinian feral pigs.
Similar content being viewed by others
Introduction
During the Neolithic Revolution, the combination of herding and agriculture induced one of the most important socioeconomic transitions in human history. Domestication techniques spread into Europe some 10 000 years ago, about 1000 years later than the first appearance of domestic species in the Fertile Crescent (Gronenborn 1999; Kuijt and Goring-Morris 2002; Colledge et al., 2004; Larson and Fuller, 2014). As part of the so-called Neolithic ‘package,’ S. scrofa represented an important prey assemblage to hunter–gatherers across wide areas of Eurasia and until the Early Holocene (Pushkina and Raia, 2008), and, once domesticated, it became economically important to farmers.
The living wild boar shows strong phylogeographic structuring in Eurasia. In Western Eurasia, S. scrofa occurs in three divergent mitochondrial DNA (mtDNA) lineages geographically segregated in continental Europe (E1), Italy (E2) and in the Near East. The European clade (E1) further shows a geographical partition in two haplogroups: A-side haplotypes are common in Central Europe and Italy, whereas C-side haplotypes are mostly found in Iberia and Eastern Europe (Giuffra et al., 2000; Larson et al., 2005; Scandura et al., 2011; Vilaça et al., 2014; Lega et al., 2016). The Near Eastern clade is traditionally characterized by the Y1 haplotype, endemic to Anatolia, and by the Y2 haplotype, whose geographical origin is currently uncertain (Evin et al., 2015; Vai et al., 2015).
During the Neolithic Revolution, humans translocated pigs into several regions across Eurasia, greatly complicating S. scrofa phylogeography. Genetic analysis of human-associated animals can be used to infer human movements and migrations. However, massive translocations, selective breeding and hybridization (Larson et al., 2007; Vigne et al., 2009) confuse patterns ascertained by using modern DNA only. Consequently, the analysis of ancient DNA (aDNA) must be relied on, in order to get direct evidence on location and timing of domestication events. The sequencing of a short mitochondrial D-loop region (80 bp) from ancient pig remains allowed, revealing that early Neolithic farmers introduced domestic pigs into Northern Europe from the Near East by the sixth millennium BC, through the so-called Danubian route. By the Bronze Age (3900 BC), European domestic pigs had replaced the Near Eastern mitochondrial haplotype (more likely through a reciprocal gene flow between local wild populations and introduced domestic stocks) and eventually spread into the Fertile Crescent (Larson et al., 2007; Evin et al., 2015; Vai et al., 2015). Domestication and dispersal of pigs into Europe following the Northern (Danubian) route is thus well documented (Larson et al., 2007; Krause-Kyora et al., 2013). In stark contrast, the identity of ancient domestic pigs in Southern Europe is still poorly known, especially so in Southern Italy, that is exactly where European Neolithic farming started (Price, 2000; Zilhao, 2001).
Southern Italy is located in a strategic geographical position in the Mediterranean basin and had a long history of human occupation and passage of different cultures since the Early Holocene. Wild boar was common and widespread in Italy in Mesolithic sites. During the Early Neolithic in Italy, farmers mainly bred cattle and goats, but pig exploitation also started soon, playing a major role (Albarella et al., 2006a, b). According to the zooarcheological record (Albarella et al., 2006a), the first S. scrofa appeared in Sardinia (and in Corsica) during the Early Neolithic, associated to human settlements and to other domestic animals, such as sheep, goat and cattle. The importance of pigs in human economy greatly increased during Iron and Roman Ages, when its frequency in archeological sites was greater than other domestic animal (MacKinnon, 2001).
European Neolithic cultures were well distinct in terms of traditions, way of life and resource exploitation. These differences persisted in the Bronze and Iron Ages, and were obviously still apparent during the Roman period. Yet, there is scarce hint about farming and husbandry in Southern regions, in comparison with Northern sites. The genetic history of Italian pigs through a very large span of time would be telling in terms of trading, origin and traditions of peopling in the Italian Peninsula.
Herein, in order to clarify domestication and dispersal of S. scrofa in these regions, we applied mtDNA analysis to 52 ancient samples, representing 15 archeological sites across Southern Italy and Sardinia, from the Mesolithic (10 000 years BC) to the Roman Age (79 AD).
Materials and methods
Sampling and dating
Bones and teeth of 52 S. scrofa specimens were collected from 15 archeological sites in Southern Italy and Sardinia, covering a period spanning from the Mesolithic (10 000 years cal BC) to the Roman Age (79 AD) (see details in Supplementary Table S1). Secure stratigraphic and/or well-defined cultural, association which provide confident relative chronology, often accompanied by indirect radiocarbon dating (Barbera et al., 1987; Malone, 2003). For more details, see Supplementary Table S1.
Morphological analysis
Although the most common method for determining the wild/domestic status of animal remains is the analysis of the postcranial bones and sizes of teeth, the reliability of these markers remains debated. In general, no single consensus technique currently exists. Unlike the size and the shape of postcranial bones, molariform teeth are much less affected by environmental factors and sexual dimorphism, and therefore significantly more reliable as indicator of wild or domestic status (Albarella and Payne, 2005). We applied several criteria to assign samples to either domestic or wild form, depending on the relative size and shape of molars and long bones, and/or where this was not possible on the cultural context and/or DNA evidence (Larson et al., 2007; Evin et al., 2014). Biometrical evidence were related to the geographic origin and the environmental effect of the study regions (Albarella et al., 2006a, b). For this study, all pre-Neolithic specimens were considered wild. Most of the samples were unequivocally classified as domestic pigs, as evident from demographic and osteometric data, and excavated from sites with secure cultural contexts of Italian sites (see Supplementary Table S1).
Authenticity criteria for the ancient DNA data
We performed cutting, surface decontamination and grinding of samples, DNA extraction and PCR processing in separate rooms dedicated to aDNA procedures, following stringent laboratory protocols tailored on aDNA processing (Cooper and Poinar, 2000). Contamination prevention included disposable coveralls, masks and gloves. All working areas and equipment were decontaminated using bleach and/or UV irradiation. Only sterile RNA/DNA-free manufactured plastic and filter tips in small packaging units were used. All reagents were molecular biology grade and, when possible, were decontaminated using UV irradiation (Fulton, 2012). Post-PCR work was conducted in facilities different from those where aDNA was processed. Negative controls were used in all experimental steps (grinding, DNA extraction, pre-PCR preparation, PCR and sequencing), to rule out the possibility of laboratory contamination. Primers applied in this study were specific for the genus Sus and showed no amplification with DNA of human or other animals either. All extractions were independently amplified and sequenced twice at least for each fragment, to rule out no misincorporated bases (Champlot et al., 2010).
DNA extraction and amplification
The surface of all samples was sanded down with a circular saw and bleached to remove contaminants. Samples were ground to fine-grained powder and DNA was extracted according a modified protocol from Yang et al., 1998. Fifty to 100 mg of powder was incubated overnight in a water bath at 56 °C in a digestion solution of 0.5 M EDTA pH 8 (Invitrogen, Carlsbad, CA, USA), 0.5% SDS and 0.1 mg ml−1 Proteinase K. DNA was extracted through silica-based spin columns (Minielute PCR Purification Kit, Qiagen, Inc., CA, USA) with a final elution volume of 50–100 μl. For each individual, a minimum of two independent DNA extractions was performed.
Following Larson et al., 2007, a 80 bp diagnostic fragment from the control region of the S. scrofa mitochondrial genome was amplified using the primers ANC1-F (5′-CTTTAAAACAAAAAAACCCATAAAAA-3′) and ANC1-R (5′-TTAATGCACGACGTACATAGG-3′). This fragment is highly variable and can distinguish between European, Near Eastern and East Asian ancient haplotypes.
Amplifications were conducted in a Mastercycler Personal (Eppendorf, Hamburg, Germany) in a 25 μl reaction volume containing: 1 × PCR Gold Buffer (Applied Biosystems, Foster City, CA, USA), 2.5 mM MgCl2, 0.2 mM each dNTP, 1.0 mg ml−1 bovine serum albumin, 0.1 μM each primer, 3.0–5.0 μl aDNA extract and 2.5 U AmpliTaq Gold DNA polymerase (Applied Biosystems). PCR was performed under the following conditions: initialization at 94 °C for 10 min; 35–45 cycles at 94 °C for 30 s, 52 °C for 30 s, 72 °C for 30 s and a final elongation step at 72 °C for 10 min. The amplification success was controlled by electrophoresis on a 1.5% agarose gel. For each extract that yielded amplifiable, sequences were obtained independently at least twice. Subsequently, 5 μl of PCR products were purified from unincorporated primers using Exonuclease I and Fast Alkaline Phosphatase (ThermoScientific, Waltham, MA, USA) in a Mastercycler at 37 °C for 30 min and at 80 °C for 15 min. The purified amplicons were directly sequenced by means of ABI Prism BigDye Terminator Cycle Sequencing Kit (ver3.1, Applied Biosystems) according to the manufacturer’s specifications. Sequencing products were purified applying the DyeEx 2.0 Spin Kit (Qiagen, Inc.) and analyzed using the ABI Prism 3100 Genetic Analyzers (Applied Biosystems).
mtDNA sequence analysis
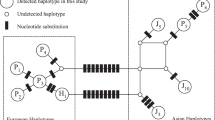
In order to identify the haplotype, the new sequences were verified and aligned manually using software Geneious version 5.4.3 (Biomatters, Auckland, New Zealand, available from http://www.geneious.com/) with pigs’ ancient haplotypes identified from across Eurasia (see Supplementary Materials in Larson et al., 2007 and Krause-Kyora et al., 2013). The 80-bp fragment of the mtDNA control region amplified by the ANC primers possesses diagnostic mutations, which allow the identification of the haplotypes of ancient specimens by visual inspection. Specifically, a transversion discriminates the ANC-Aside from the ANC-Cside. The addition or subtraction of a base is enough to visually discriminate between the Near Eastern haplotypes Y1 and Y2. Other three characteristic polymorphisms distinguish the ANC-Italy haplotypes from the others (see Supplementary Table S2). Haplotypes were then collapsed using the DnaSP version 5 software (Librado and Rozas, 2009) and a three-dimensional phylogenetic network was constructed with the software Network 4.5.1.6 (Bandelt et al., 1999) (Fluxus Technology, http://www.fluxus-engineering.com/; Figure 1b). The list of haplotypes are shown in Supplementary Table S3. DNA sequences have been deposited in GenBank (http://www.ncbi.nlm.nih.gov/), with accession numbers KT321849–KT321851 and KT321853).
(a) Distribution of mtDNA haplotypes in wild boar and pig ancient specimens in Southern Italy and Sardinia. Different shapes depict five approximate sample ages: Mesolitic (10 000–5500 BC), Neolithic (5500–3900 BC), Bronze Age (3900–700 BC), Iron Age (700 to first century BC), Roman Age (first century BC to fourth century AD). Distinct haplotypes are represented by different colors: green (European E1 A-side), blue (European E2 C-side), brown (Italian E2) and yellow (Near Eastern Y1). Asterisks mark ancient specimens classified as ‘wild.’ Symbols without contour refer to ancient samples reported by Larson et al., 2007. The map was generated with the software ArcGis v.9.1.3. (b) Three-dimensional phylogenetic network based on haplotypes identification of the 80 bp fragment of the mtDNA control region of Sus scrofa. Layers represent the haplotypes of ancient Southern Italian and Sardinia samples in five consecutive time periods. Each circle represents one haplotype. Distinct haplotypes correspond to different colors: green (European E1 A-side), blue (European E2 C-side), brown (Italian E2) and yellow (Near Eastern Y1). Small black dots represent missing haplotypes. The size of the circles is proportional to the number of haplotype found. Vertical lines link haplotypes in time periods.
Verification of mtDNA results
In order to verify the authenticity of the data obtained in the Department of Biology in Naples (Italy), 20% of samples were analyzed in the independent laboratory of the University of Tel Aviv, Israel (see Supplementary Table S1). DNA extractions and preparations for PCR reactions of the ancient samples were set up at the Institute of Archaeology of Tel Aviv University (Israel) in a dedicated lab for ancient DNA, in a building where no molecular work has previously taken place or is currently being conducted. DNA from bones was extracted according to a method modified from Yang et al., 1998. Specifically, ~50 mg of bone powder was incubated with 0.44 M EDTA pH 8 (AMRESCO, Solon, OH, USA), 0.1 M urea and 20 mg ml−1 proteinase K (AMRESCO) overnight at 56 °C. After decalcification and digestion, the supernatant was concentrated to about 100 μl using Vivaspin filter 3000 MWCO (Sartorius Stedim Biotech, Aubagne, France) and then directly purified using silica-based spin columns (Minelute PCR Purification kit, Qiagen, Inc.) with a final elution volume of 70 μl. PCRs and post-PCR work were performed at the Zoology Department of Tel Aviv University (Israel). The 80 bp diagnostic fragment from the control region of the S. scrofa mitochondrial genome was amplified using the primers ANC1 (Larson et al., 2007). PCR amplifications were performed in 25 μl reactions with the following: 1 × PCR buffer, 1.5 U of Platinum Taq DNA polymerase High Fidelity, 2 mM MgSO4, 0.2 mM of each dNTP (all Invitrogen, Glasgow, UK), 0.1 mg ml−1 bovine serum albumin (New England BioLabs, Hitchin, UK), 0.4 μM of each primer (Sigma-Aldrich, St Louis, MO, USA) and 2–4 μl of extract. The PCR amplification consisted of an initial denaturing at 94 °C for 4 min, followed by 55 cycles of denaturing at 94 °C for 30 s, annealing at 52 °C for 30 s and extension at 68 °C for 40 s with a final extension period of 5 min at 68 ºC. The amplicons were cleaned from unincorporated primers using Exonuclease I and Shrimp Alkaline Phosphatase (Thermo Fisher Scientific, Renfrew, UK). The samples were sequenced and analyzed in ABI 300 Genetic Analyzer, Perkin-Elmer, Foster City, CA, USA. Sequencing was conducted on both strands.
Results
We successfully extracted and sequenced DNA from 27 out of 52 ancient S. scrofa remains from 15 archaeological sites in Southern Italy and Sardinia. All samples were re-extracted and amplified independently at least twice. Only those sequences resulting in the same haplotype from independent experiments were considered in the phylogenetic analysis (see Supplementary Table S1). The overall success rate was 52%, which represents an unexpectedly high rate given the predominant climate of the region. Fortunately, low levels of humidity typical of cave environments, where most of the samples were found, probably protected DNA from excessive degradation (Poinar et al., 2003).
The mtDNA sequences from the specimens recovered from Southern Italian (n=21) and Sardinian (n=6) sites cluster into four previously described wild boar haplotypes (E1-A, E1-C, E2-Italy and NE-Y1; Figure 1a and also see Supplementary Table S1). To examine the temporal changes in haplotype distribution, we grouped the results into five cultural time periods corresponding to Italian Mesolithic (n=9), Neolithic (n=2), Bronze Age (n=8), Iron Age (n=3) and Roman Age (n=5; Figure 1).
Seven Mesolithic specimens belong to the European C-side haplotype and two further to the European A-side haplotype. The two Neolithic samples from Sardinia belong to the European A- and C-side haplotypes, respectively.
Peninsular samples tracing back to the Bronze Age (n=4) show European haplotypes. A further, Late Bronze-age domestic specimen belongs to to the Italian clade. Interestingly, all of the three domestic pigs from the Bronze Age coming from the major Italian islands (Sardinia and Sicily) possess the Near Eastern haplotype Y1. We analyzed three Iron Age samples from Sardinia. They all belong to the E1 clade. In particular, two samples pertain to the C-side haplotype and one to the A-side. All Roman-aged samples possess the European A-side haplotype, except for the pig from Boscoreale (79 AD) that shows the C-side haplotype.
Discussion
Ancient S. scrofa remains analyzed in this study shed new light on the geographic and temporal distribution of this species in Southern Italy and Sardinia, revealing new and important insights about past human migrations in the Mediterranean basin.
The analysis of Mesolithic samples shows the presence of European A-side haplotype in Southern Italy (Grotta dell’Ausino, Campania) as early as during the Mesolithic. This very result shifts the presence of such particular clade in Italy 6,500 years back in time (cf. Larson et al., 2007, 1500 BC). It further expands the geographic range of Mesolithic A-side wild boar to peninsular Italy. Data obtained from Mesolithic samples also confirm the presence of wild boar European C-side haplotype in Southern Italy (Latronico) and add novel information about the C-side presence in Sicily, which possibly took place during moments of sea-level low stands during the latest Pleistocene. These data also represent the first genetic characterization of ancient Sus samples in Sicily, for which no evidence was published so far. The presence of the two haplotypes in Southern Italy (E1 A-side and C-side) together with previously published Mesolithic data (the Italian E2 haplotype reported in Larson et al., 2007) further points to the high variability of mtDNA of this geographical region before the domestication and the human manipulation. This bolsters the idea that southernmost Europe acted as a genetic reservoir for the species during the last glaciation (Hewitt, 2004; Alexandri et al., 2012; Vilaça et al., 2014).
Regarding the Neolithic, Sardinian sequences represent the oldest genetic information ever retrieved for any major Mediterranean islands for the wild boar. The fossil record of insular pigs suggests they are poor swimmers; hence, their association with livestock in Sardinia and Corsica strongly indicates human introduction (Albarella et al., 2006a, b). Domestic pigs with European signatures were therefore probably imported on the island during the Sardinian Late Neolithic (corresponding to the European Bronze Age), when the replacement of Near Eastern haplotypes with those European in domestic remains was already advanced. However, the absence of Near Eastern haplotype Y1 in Italian samples is possibly related to the limited number of S. scrofa bones and teeth, which are overall rare in Neolithic European sites (Tresset and Vigne, 2001). Future studies are necessary in order to understand the spread of domestic pigs in Italy during the Neolithic Revolution.
Our data on Bronze Age S. scrofa surprisingly reveal that Near Eastern Y1 haplotype was present on both the two major Italian islands. This could be either because Y1 individuals were already there but never found before or humans exploiting this particular strain at least during the Bronze Age brought them to the islands. The first scenario is consistent with natural dispersion from other Italian areas (such as it is the case for Biarzo in Northern Italy, where pigs’ remains belong to the Y2 haplotype; Vai et al., 2015). However, unlike the Y2 haplotype, which occurs in several pre-Neolithic European contexts (Evin et al., 2015, Vai et al., 2015) and in modern feral pigs from Corsica (Larson et al., 2007), the Y1 haplotype was never found in neither Mesolithic or modern European wild boar. Natural dispersion of pigs over large sea stretches presumes pigs were good swimmers, which is not the case. In fact, it is remarkable that S. scrofa is thoroughly absent form late Pleistocene insular faunas unrelated to human occupation (Reyment, 1983, Raia and Meiri, 2006). Thus, the possibility of natural dispersion to the islands appears implausible. Alternatively, pigs made for the two islands during the Bronze Age, thanks to human translocation. Rapid demographic and cultural changes took place during the Bronze (1600–1200 BC) and ensuing Iron Ages (1200–600 BC), including large-scale migrations of people, the establishment of exchange networks across the Mediterranean (Dyson and Rowland, 2007) and a gradual improvement of pigs’ importance to human economy (MacKinnon, 2001; Albarella et al., 2006a, b). It is conceivable that Near Eastern pigs were imported in Sicily and Sardinia at least around 1600–1300 BC. At that time, the island of Sardinia was characterized by the Nuragic Civilization and showed a strong connection to the Sea People during the thirteenth century BC and a separation with the peninsular cultures. Nuragic ceramics were also found in Sicily (Castelluccio culture) and along the sea routes to the Eastern Mediterranean (for example, Crete; Balmuth, 1987). Our hypothesis is that the presence of the Near Eastern haplotypes in the two Italian islands connects to the trading activities of the Sea People in the Mediterranean basin at that time with the Nuragic and Castelluccio cultures (Tykot, 1994). The same people may have later intentionally dispersed the European haplotype into the Levant during the Iron Age. Indeed, the major turnover in the Near Eastern pig population took place around 900 BC, when the European haplotype became predominant in the Southern Levant (Meiri et al., 2013), in Anatolia (Ottoni et al., 2013) and Romania (Evin et al., 2015). A similar scenario has been proposed for the Eastern Mediterranean origin of cattle in Etruria (Central Italy) introduced in this region by navigation during the Late Bronze (Pellecchia et al., 2007).
The domestic sample from the Bronze Age carrying the Italian E2 signature reveals new information about the geographic distribution and the domestication of this particular clade. The endemic Italian clade has been previously found only in ancient Italian and Croatian specimens (Larson et al., 2007, Vai et al., 2015). Currently, the E2 clade is observed only in Central/Southern Italian wild boar and Sardinian feral populations, but never in domestic pigs (Scandura et al., 2008; Alexandri et al., 2012; Vilaça et al., 2014). The presence of endemic Italian clade in Southern Italy (Poggiomarino, Campania) in more recent domestic contexts support the hypothesis that the indigenous Italian wild boar was effectively domesticated, or that female Italian wild boar were incorporated into domestic stocks in this region during the Bronze Age. Our data suggest that domestic pigs with Italian signature were imported to Sardinia by at least the end of the second millennium BC through sea trading between the Peninsula and the island cultures. Sardinian feral pigs carrying E2 haplotypes are still present today and may represent the last-standing genetic evidence of the domestication of the endemic Italian wild boar.
Finally, our data provide evidence for the A-C side mtDNA turnover occurred in European domestic pig populations. The A-side haplotype showed higher relative frequency in domestic remains since the Neolithic, at the expense of C-side (Larson et al., 2007, Lega et al., 2016). We confirmed that the A-side haplotype individuals seem to have been preferentially domesticated or at least more abundant in domestic contexts in Italy (Figure 1b).
In conclusion, a number of new insights emerge from this study. The history and economy of Italy’s two major islands during the Bronze and Iron Ages was certainly influenced by the dispersal of domestic pigs occurred by navigation, although we do not know precisely to what extent. The processes of pig domestication and diffusion in Italy were certainly complex and our data contribute significantly, in terms of the number of ancient mtDNA sequences generated, to the genetic history of pigs in Italy. Despite the well-known methodological challenges, our results further prove the power of ancient DNA in revealing past human activities, trades and dispersion of domesticated species.
The conclusions of this study are based on a mitochondrial locus, which only detects the matrilineal inheritance and it is a poor predictor of overall genomic diversity (Bruford et al., 2003). The analysis of nuclear markers, including the Y-chromosome for paternal inheritance (Ramirez et al., 2009) and the MC1R gene as a marker of hybridization wild/domestic (Fang et al., 2009), will undoubtedly reveal a clearer phylogenetic pattern.
Data Archiving
Sequence data have been submitted to GenBank: accession numbers KT321849–KT321851 and KT321853. The supporting information is available through the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.cv6n5.
References
Albarella U, Payne S . (2005). Neolithic pigs from Durrington Walls, Wiltshire, England: a biometrical database. J Archaeol Sci 32: 589–599.
Albarella U, Manconi F, Rowley-Conwy P, Vigne JD . (2006a) Pigs of Corsica and Sardinia: a Biometrical Re-evaluation of their Status and History in Archaeozoological Studies in Honour of Alfredo Riedel. In: Tecchiati U, Sala B (eds), Province of Bolzano: Bolzano, Italy, pp 285–302.
Albarella U, Tagliacozzo A, Dobney K, Rowley-Conwy P . (2006b). Pig hunting and husbandry in prehistoric Italy: a contribution to the domestication debate. Proc Prehist Soc 72: 193–227.
Alexandri P, Triantafyllidis A, Papakostas S, Chatzinikos E, Platis P, Papageorgiou N et al. (2012). The Balkans and the colonization of Europe: the post-glacial range expansion of the wild boar Sus scrofa. J Biogeogr 39: 713–772.
Balmuth MS . (1987) Studies in Sardinian Archaeology III: Nuragic Sardinia and the Mycenaean World. British Archaeological Reports International Series. Oxford: British Archaeological Reports. pp 7–37.
Bandelt HJ, Forster P, Röhl A . (1999). Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16: 37–48.
Barbera C, Conte A, Virgili A . (1987) Prime osservazioni sulle mammalofaune della grotta dell’Ausino (Sa) ‘Notiziario Sezionale C.A.I’ n. 3 pp 31–35.
Bruford MW, Bradley DG, Luikart G . (2003). DNA markers reveal the complexity of livestock domestication. Nat Rev Genet 4: 900–910.
Champlot S, Berthelot C, Pruvost M, Bennett EA, Grange T, Geigl E-M . (2010). An efficient multistrategy DNA decontamination procedure of PCR reagents for hypersensitive PCR applications. PLoS ONE 5: e13042.
Colledge S, Conolly J, Shennan S . (2004). Archaeobotanical evidence for the spread of farming in the eastern Mediterranean. Curr Anthropol 45: S35–S58.
Cooper A, Poinar HN . (2000). Ancient DNA: do it right or not at all. Science 289: 1139.
Dyson SL, Rowland RJ . (2007) Shepherds, Sailors, & Conquerors - Archeology and History in Sardinia from the Stone Age to the Middle Ages. University of Pennsylvania, Museum of Archeology and Anthropology: Philadelphia.
Evin A, Cucchi T, Escarguel G, Owen J, Larson G, Vidasdottir US et al. (2014). Using traditional biometrical data to distinguish West Palearctic wild boar and domestic pigs in the archaeological record: new methods and standards. J Archaeol Sci 43: 1–8.
Evin A, Flink LG, Bălăşescu A, Popovici D, Andreescu R, Bailey D et al. (2015). Unravelling the complexity of domestication: a case study using morphometrics and ancient DNA analyses of archaeological pigs from Romania. Phil Trans R Soc B 370: 20130616.
Fang M, Larson G, Ribeiro HS, Li N, Andersson L . (2009). Contrasting mode of evolution at a coat color locus in wild and domestic pigs. PLoS Genet 5: e1000341.
Fulton TL . (2012). Setting up an ancient DNA laboratory. Methods Mol Biol 840: 1–11.
Giuffra E, Kijas JM, Amarger V, Carlborg O, Jeon JT, Andersson L . (2000). The origin of the domestic pig: independent domestication and subsequent introgression. Genet Soc Amer 154: 1785–1791.
Gronenborn D . (1999). A variation on a basic theme: the transition to farming in southern Central Europe. J World Prehist 2: 23210.
Hewitt GM . (2004). Genetic consequences of climatic oscillations in the Quaternary. Phil Trans R Soc Lond B 359: 183–195.
Krause-Kyora B, Makarewicz C, Evin A, Flink LG, Dobney K, Larson G et al. (2013). Use of domesticated pigs by mesolithic hunter-gatherers in Northwestern Europe. Nat Commun 4: 1–7.
Kuijt I, Goring-Morris N . (2002). Foraging, farming, and social complexity in the pre-pottery Neolithic of the southern Levant: a review and synthesis. J World Prehist 16: 361–440.
Larson G, Dobney K, Albarella U, Fang M, Matisoo-Smith E, Robins J et al. (2005). Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science 307: 1618–1621.
Larson G, Albarella U, Dobney K, Rowley-Conwy P, Schibler J, Tresset A et al. (2007). Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. Proc Natl Acad Sci USA 104: 15276–15281.
Larson G, Fuller DQ . (2014). The evolution of animal domestication. Annu Rev Ecol Evol 45: 115–136.
Lega C, Raia P, Rook L, Fulgione D . (2016). Size matters: a comparative analysis of pig domestication. Holocene 26: 327–332.
Librado P, Rozas J . (2009). DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452.
MacKinnon M . (2001). High on the Hog: linking zooarchaeological, literary, and artistic data for pig breeds in Roman Italy. Am J Archaeol 105: 649–673.
Malone C . (2003). The Italian Neolithic: a synthesis of research. J World Prehist 17: 3.
Meiri M, Huchon D, Bar-Oz G, Boaretto E, Horwitz LK, Maeir AM et al. (2013). Ancient DNA and population turnover in southern levantine pigs- signature of the sea peoples migration? Sci Rep 3: 3035.
Ottoni C, Flink LG, Evin A, Geörg C, De Cupere B, Van Neer W et al. (2013). Pig domestication and human-mediated dispersal in western Eurasia revealed through ancient DNA and geometric morphometrics. Mol Biol Evol 30: 824–832.
Pellecchia M, Negrini R, Colli L, Patrini M, Milanesi E, Achilli A et al. (2007). The mystery of Etruscan origins. Proc R Soc London Ser B 274: 1175–1179.
Poinar HN, Kuch M, McDonald G, Partin P, Pääbo S . (2003). Genetic analyses from ancient DNA. Curr Biol 13: 1150–1152.
Price TD . (2000) Europe’s First Farmers. Cambridge University Press.
Pushkina D, Raia P . (2008). Human influence on distribution and extinctions of the late Pleistocene Eurasian megafauna. J Hum Evol 54: 769–782.
Raia P, Meiri S . (2006). The island rule in large mammals: paleontology meets ecology. Evolution 60: 1731–1742.
Ramirez O, Ojeda A, Tomas A, Gallardo D, Huang LS, Folch JM et al. (2009). Integrating Y-chromosome, mitochondrial and autosomal data to analyse the origin of pig breeds. Mol Bio Evol 26: 2061–2072.
Reyment RA . (1983). Palaeontological aspects of island biogeography: colonization and evolution of mammals on Mediterranean islands. Oikos 41: 299–306.
Scandura M, Iacolina L, Crestanello B, Pecchioli E, Di Benedetto MF, Russo V . (2008). Ancient vs. recent processes as factors shaping the genetic variation of the European wild boar: are the effects of the last glaciation still detectable? Mol Ecol 1: 1745–1762.
Scandura M, Iacolina L, Apollonio M . (2011). Genetic diversity in the European wild boar (Sus scrofa: phylogeography, population structure and wild x domestic hybridization. Mamm Rev 41: 125–137.
Tresset A, Vigne JD . (2001) La chasse, principal élément structurant la diversité des faunes archéologiques du Néolithique ancien, en Europe tempérée comme en Méditerranée in: Arbogast RM, Jeunesse C, Schibler J eds Rôle et statut de la chasse dans le Néolithique ancien danubien (5500–4900 av. J.-C.). Marie Leidorf: Rahden, Germany. pp 129–151.
Tykot RH . (1994). Sea peoples in Etruria? Italian contacts with the Eastern Mediterranean in the Late Bronze Age. Etruscan Stud 1: 59–83.
Vai S, Vilaça ST, Romandini M, Benazzo A, Visentini P, Modolo M et al. (2015). The Biarzo case in northern Italy: is the temporal dynamic of swine mitochondrial DNA lineages in Europe related to domestication? Sci Rep 5: 16514.
Vigne JD, Zazzo A, Saliege JF, Poplin F, Guilaine J, Simmons A . (2009). Pre-Neolithic wild boar management and introduction to Cyprus more than 11,400 years ago. Proc Natl Acad Sci USA 106: 16131–16138.
Vilaça ST, Zachos F, Biosa D, Iacolina L, Kirschning J, Alves PC et al. (2014). Mitochondrial phylogeography of the European wild boar: the effect of climate on genetic diversity and spatial lineage sorting across Europe. J Biogeogr 41: 987–998.
Yang DY, Eng B, Waye JS, Dudar JC, Saunders SR . (1998). Technical note: improved DNA extraction from ancient bones using silica-based spin columns. Am J Phys Anthropol 105: 539–543.
Zilhao J . (2001). Radiocarbon evidence for maritime pioneer colonization at the origins of farming of west Mediterranean Europe. Proc Natl Acad Sci USA 98: 14180–14185.
Acknowledgements
We are grateful to Dr Ernesto De Carolis and the ‘Soprintendenza Speciale per i Beni archeologici di Pompei Ercolano Stabia,’ Dr Annamaria Sodo and the ‘Antiquarium di Boscoreale,’ Professor Barbara Wilkens (University of Sassari, Sardinia) and the ‘Soprintendenza delle Province di Sassari e Nuoro’ for granting permits and the analysis of the samples necessary to conduct this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Heredity website
Supplementary information
Rights and permissions
About this article
Cite this article
Lega, C., Fulgione, D., Genovese, A. et al. Like a pig out of water: seaborne spread of domestic pigs in Southern Italy and Sardinia during the Bronze and Iron Ages. Heredity 118, 154–159 (2017). https://doi.org/10.1038/hdy.2016.74
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2016.74
This article is cited by
-
Reprogramming of the gut microbiota following feralization in Sus scrofa
Animal Microbiome (2023)
-
Mitochondrial DNA diversity of the Sardinian local cattle stock
Scientific Reports (2022)
-
Eastern Mediterranean Mobility in the Bronze and Early Iron Ages: Inferences from Ancient DNA of Pigs and Cattle
Scientific Reports (2017)