Abstract
The genetic basis of host preference has been investigated in only a few species. It is relevant to important questions in evolutionary biology, including sympatric speciation, generalist versus specialist adaptation, and parasite–host co-evolution. Here we show that a major locus strongly influences host preference in Nasonia. Nasonia are parasitic wasps that utilize fly pupae; Nasonia vitripennis is a generalist that parasitizes a diverse set of hosts, whereas Nasonia giraulti specializes in Protocalliphora (bird blowflies). In laboratory choice experiments using Protocalliphora and Sarcophaga (flesh flies), N. vitripennis shows a preference for Sarcophaga, whereas N. giraulti shows a preference for Protocalliphora. Through a series of interspecies crosses, we have introgressed a major locus affecting host preference from N. giraulti into N. vitripennis. The N. giraulti allele is dominant and greatly increases preference for Protocalliphora pupae in the introgression line relative to the recessive N. vitripennis allele. Through the utilization of a Nasonia genotyping microarray, we have identified the introgressed region as 16 Mb of chromosome 4, although a more complete analysis is necessary to determine the exact genetic architecture of host preference in the genus. To our knowledge, this is the first introgression of the host preference of one parasitoid species into another, as well as one of the few cases of introgression of a behavioral gene between species.
Similar content being viewed by others
Introduction
The genetic basis of host preference is relevant to a number of fundamental evolutionary questions. These include evolution of specialization and generalization (Whitlock 1996; Kelley and Farrell, 1998), sympatric speciation (Rice 1987; Dieckmann and Doebelli 1999; Kondrashov and Kondrashov, 1999), host shifts (Knowles et al., 1999; Groman and Pellmyr, 2000) and parasite–host and plant–herbivore co-evolution (Futuyma and Mitter, 1996; Forbes et al., 2009). For example, the presence of host choice can drive the evolution of specialization, as organisms adapt to the hosts to which they are more frequently exposed (Whitlock 1996; Kawecki 1998). In speciation models, the simpler the genetic basis of host preference and performance, and the more these traits are tightly linked, the more likely sympatric speciation is to occur (Fry 2003). An additional consideration is that parasitoids are widely used for the biological control of pests of agricultural importance (Quicke, 1997). A better understanding of the genetics of host range in parasitoids could also facilitate genetic improvement of these insects in biological pest control, by providing mechanisms for genetic manipulation of host usage.
Host selection behavior involves several phases, including habitat location, host location, host recognition and host acceptance (Jaenike, 1990; Vinson, 1998). All these stages may be under both genetic and environmental influence (Geervliet et al., 1998). However, the genetic basis of these behaviors has been investigated in only a few systems. Most of the work on host selection behavior in arthropods has been done in phytophagous insects (reviewed by Jaenike, 1990) parasitic hymenoptera (reviewed by Vinson, 1998), and ticks and mites (Magalhães et al., 2007). Genetic studies have suggested a wide variation between systems in the number of loci and mode of inheritance involved in host preference (for example, Jaenike, 1987; Thompson et al., 1990; Keese, 1996; Messina and Slade, 1997; Tucic et al., 1997; Hawthorne and Via, 2001; Nylin et al., 2005).
The most well-studied genetic system for host preference is that of Drosophila sechellia, a species of Drosophila endemic to the Seychelles Islands which feeds solely on the fruit of Morinda citrifolia, which is toxic to most other Drosophila species (Louis and David, 1986; Jones, 2005). It was subsequently shown that two genes encoding odorant binding proteins affect the species’ responses to hexanoic and octanoic acid, and therefore their attraction to the fruit (Matsuo et al., 2007). Knockout of one of these genes in Drosophila melanogaster, Obp56e, caused the flies to lose much of their aversion to morinda fruit (Dworkin and Jones, 2009). In aphids, Hawthorne and Via 2001) found a complex basis to host preference, with several groups of tightly linked quantitative trait loci involved in host choice and fitness.
The present work analyzes the genetics of host preference differences in the parasitic wasp Nasonia. There are four species of Nasonia: Nasonia vitripennis, Nasonia giraulti, Nasonia longicornis and the newly described Nasonia oneida (Raychoudhury et al., 2010). The species are interfertile once cured of Wolbachia (Breeuwer and Werren, 1990, 1995), allowing traits from one species to be introgressed into another (Weston et al., 1999; Loehlin et al., 2010). With the recent sequencing of the genomes of three species (Werren et al., 2010) and a wealth of other resources becoming available (for example, Lynch and Desplan, 2006; Niehuis et al., 2010; Pannebakker et al., 2010), Nasonia provides a powerful system for studying the genetic basis of interspecies differences (Werren and Loehlin, 2009).
Nasonia consists of both a generalist and specialist species. N. vitripennis has a holarctic distribution and is a generalist that parasitizes a wide range of calyptrate flies, including blowflies, house flies and flesh flies. N. giraulti and N. longicornis specialize on bird blowflies (Protocalliphora, which N. vitripennis also parasitizes) and occur in northeastern and northwestern parts of North America, respectively (Darling and Werren, 1990). The newly described N. oneida also specializes in bird blowflies and is, at present, known to occur only in upstate New York, USA (Raychoudhury et al., 2010). N. vitripennis, N. giraulti and N. oneida occur microsympatrically in bird nests in eastern North America, whereas N. vitripennis is also found associated with carrion-breeding flies.
As host choice is hypothesized to drive the evolution of specialization (Whitlock 1996; Kawecki 1998), the genetics of host preference is particularly relevant to the evolution of host usage in Nasonia. Evidence of a host preference locus was originally detected during the introgression of male-specific wing-size locus ws1g from N. giraulti into N. vitripennis (Weston et al., 1999). Pure breeding of the line with ws1g in a largely N. vitripennis genetic background was difficult because homozygous females did not sting the Sarcophaga (flesh fly) hosts that are used for the maintenance of wasp strains in the laboratory and are regular hosts of N. vitripennis in the wild. A pilot experiment indicated that they did sting Protocalliphora hosts. Subsequently, ws1g was separated from these effects by recombination, allowing purebreeding of ws1g (Weston et al., 1999). Here, we backcross the region around ws1g from N. giraulti into N. vitripennis using newly available visible markers, and map a major host-preference effect in the region.
Materials and methods
Nasonia strains and maintenance
The general biology of Nasonia is described by Whiting (1967). Cultures of Nasonia were maintained in the laboratory with constant light and temperature (25 °C) on Sarcophaga pupae. Under these conditions the generation time is approximately 14 days. For laboratory experiments on host preference and acceptance, the standard reference strains of N. vitripennis (ASymCx) and N. giraulti (RV2Xu) were used (Werren et al., 2010). To introgress (backcross) the region around ws1g from N. giraulti into N. vitripennis, we used the mutant N. vitripennis strain peach (pe333). Previous studies had revealed that this eye color mutant interacts epistatically with a natural eye color allele in N. giraulti (bkg) that is linked to ws1g, thus permitting easy tracking of the region during backcrossing.
For host preference experiments we used Sarcophaga bullata and Protocalliphora sialia pupae. A Sarcophaga culture was maintained in the laboratory, whereas Protocalliphora were obtained as larvae from bluebird and tree swallow nests during the summer months. The larvae were separated and allowed to pupate. Two days after pupation, both Sarcophaga and Protocalliphora were placed in a refrigerator at 4 °C, where they were stored for upto 4 weeks before their use in experiments.
Host-acceptance tests of field-collected wasps
To test for the acceptance of Sarcophaga hosts by field-collected wasps, we collected bluebird and tree swallow nests from eight different states in the eastern and midwestern parts of United States of America (New York, Ohio, Virginia, Pennsylvania, Indiana, Minnesota, Michigan and Wisconsin). Wasps were allowed to emerge from the nests in the laboratory, and we collected females from those nests that contained either all N. giraulti (14 nests) or all N. vitripennis (102 nests). To assess acceptance of Sarcophaga hosts, the females were placed in a vial with one Sarcophaga pupa and allowed to parasitize until the wasp dies (approximately 3–6 days). Two to three weeks later, hosts were scored for the presence of adult flies or wasps (adults or diapausing larvae).
Host preference and acceptance experiments
Observations were carried out to characterize the behavioral response of Nasonia strains to Sarcophaga and Protocalliphora pupae. Virgin females, 2–3 days old, were placed in individual vials. Each female was given either one Sarcophaga host and one Protocalliphora host in a host preference (that is, choice) experiment or two Sarcophaga in a host acceptance experiment. Vials were set horizontally so that the female's behavior could be observed. The female's contact with and stinging of hosts was recorded. A ‘contact’ was recorded if the female was on the host but not stinging it. A ‘sting’ was recorded if the observer could see the ovipositor probing/stinging the host. Observations were made every 5 min for the first hour, every 10 min for the second hour and subsequently every 15 min. Observations ceased approximately 4.5 h after the female was first given the host. Each wasp was then scored for (1) whether or not it contacted a host at all during observation (contact), (2) how much time it spent contacting each host (time spent on host) and (3) whether or not it stung during observation (stinging). After 24 h, each host was removed and scored two to three weeks later for the presence of adult flies or wasps (adults or diapausing larvae). Statistical comparisons were carried out using contingency χ2 tests or Mann–Whitney U tests. Both host acceptance and preference tests were carried out on N. vitripennis (strains ASymCx and peach), N. giraulti (strain RV2Xu) and heterozygous bkbwg/+v introgression females (described below).
Introgression of the bkbwg region into N. vitripennis
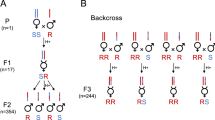
To test for host preference effects in the ws1g region, we introgressed (backcrossed) the region around ws1 from N. giraulti into an N. vitripennis genetic background. We hereafter refer to this region as bkbwg (for black eyes, big wings and naturally occurring N. giraulti visible markers in the region). Specifically, the bkbwg region contains the visible markers ws1g, swwg, and bkg (see bottom of Figure 1). These visible markers, described below in more detail, allow heterozygous bkbwg/+v females to be visibly distinguished from homozygous bkbwg/bkbwg females and bkbwg males to be visibly distinguished from +v males.
Generation of the bkbwg (for black eyes, big wings and naturally occurring N. giraulti visible markers in the region) introgression line and map of visible markers in the region. In the parental cross, Nasonia giraulti females were mated to Nasonia vitripennis peach males. In each backcross generation, heterozygous females, identified by visible markers (m) in the bkbwg region, were mated to Nasonia vitripennis peach males to further reduce the size of the introgression. To purebreed the line, heterozygous bkbwg/+v females were mated to bkbwg introgression males, and their homozygous bkbwg/bkbwg introgression female offspring were again mated to bkbwg introgression males in an attempt to produce an isogenic line. Inset is a map of the bkbwg region, including the location of visible markers ws1 (wing size 1), bl13 (blue 13), bk576 (black 576) and sww (shorter wider wings). Markers indicated with asterisks were used to track the bkbwg introgression. Distances between visible markers are shown in centiMorgans (cM).
The process of the introgression is outlined in Figure 1. Initially, N. giraulti females were crossed to N. vitripennis peach males (Figure 1, parental). F1 hybrid females were then backcrossed to N. vitripennis peach males (Figure 1, backcross). Each backcross generation, hybrid heterozygous bkbwg/+v females, identified using visible markers in the region (Figure 1), were mated to N. vitripennis peach males. After 10 generations, an attempt was made to produce a homozygous bkbwg strain by crossing bkbwg hybrid males to heterozygous bkbwg/+v females (Figure 1, purebreed). In the next generation, all females were mated to bkbwg males. The incidence of failure to parasitize Sarcophaga hosts increased dramatically, and nearly all females parasitizing hosts proved to be bkbwg/+v heterozygotes rather than bkbwg/bkbwg homozygotes. This made the production of a purebred bkbwg introgression line impossible. It is to be noted that these hybrid incompatibilities are only seen in the purebreeding stage and not in the late-generation backcrosses, because hybrid incompatibilities in Nasonia tend to be recessive (Breeuwer and Werren, 1995). The bkbwg strain is therefore maintained heterozygously, by crossing heterozygous bkbwg/+v females with bkbwg males.
Visible markers in the bkbwg region
Integrity of the bkbwg introgression strain is maintained using visible markers (a map of these markers is shown at the bottom of Figure 1). The region maps onto linkage group IV (chromosome 4), and on one end lies the major male wing size QTL ws1 (Weston et al., 1999, Werren et al., 2010). Approximately 0.74 cM from ws1 is the mutant body color allele bl13, which causes a blue-colored body. This N. vitripennis mutation was originally generated by Saul et al. (1965), but had been mapped incorrectly to linkage group III (Saul et al., 1967). On the opposite side of bl13, 0.09 cM away, lay the loci bk and sww. The bk and sww loci were discovered during experiments conducted to introgress additional wing size QTL, using a N. vitripennis strain (peach) with the R-locus mutant pe333 on chromosome 5 which causes ‘peach’ colored eyes (JH Werren and L Enders, unpublished data). These experiments revealed an epistatic interaction between pe333 and N. giraulti wild-type eye locus bkg, which creates ‘oyster’-colored eyes when both markers are homozygous. Epistatic effects among some eye color mutants generated in N. vitripennis had been previously observed (Saul and Kayhart, 1956), apparently involving mutants in the red versus brown pigment pathways. Subsequent genetic analyses revealed that the natural N. giraulti eye color effect was allelic to the N. vitripennis locus bk576 on linkage group IV. A mutant at bk576 causes blackish eyes (Saul et al., 1965). Introgression males showing the oyster eye phenotype also had large wings, even larger than the standard ws1g males. The wing size effects within the region are due to introgression of N. giraulti alleles at ws1 and a second locus, called shorter wider wings (sww). Analyses of sww will be reported elsewhere.
Mapping of the bkbwg region using a genotyping microarray
To ascertain the size and content of the bkbwg region, we used a genotyping microarray that has been developed to genotype hybrids between N. vitripennis and N. giraulti. Recent sequencing of the genome of N. vitripennis and partial sequencing of N. giraulti (Werren et al., 2010) has identified an abundance of interspecies polymorphisms. N. giraulti reads were aligned to the N. vitripennis genome sequence, and single-nucleotide polymorphisms, small insertions and deletions were used to design oligonucleotide probes to discriminate between N. vitripennis and N. giraulti DNA (Werren et al., 2010). Probes for >19 000 loci, covering 929 scaffolds and 86% of the assembled genome, were printed on custom NimbleGen microarrays (Madison, WI, USA). The details of the microarray will be described elsewhere (Desjardins CA et al., unpublished).
Deoxyribose nucleic acid was prepared from a single bkbwg introgression male using a Puregene Gentra DNA extraction kit (Qiagen, Valencia, CA, USA) using the protocol for a single Drosophila (http://www1.qiagen.com/Products/GenomicDnaStabilizationPurification/GentraPuregeneCellKit.aspx). This DNA was subsequently amplified via multiple displacement amplification using an Illustra Genomiphi V2 kit (GE Healthcare, Piscataway, NJ, USA). This DNA was labeled and hybridized to the array according to described protocols (Werren et al., 2010). After hybridization, each locus on the array was identified as N. vitripennis, N. giraulti or ambiguous. We then examined the loci in all major (>350 Kb) scaffolds (contiguous DNA sequences) on chromosome 4 to determine the genotypes of the scaffolds.
Results
N. vitripennis and N. giraulti differ in host preference
In order to assess differences in host acceptance between Nasonia species, N. giraulti and N. vitripennis wasps that had emerged from field collected birds nests were tested for acceptance rates of Sarcophaga pupae. Whereas 83% (2260 of 2739) of N. vitripennis females stung Sarcophaga hosts, only 45% (184 of 410) of N. giraulti females did so (χ21=291, P<0.001). Therefore, field-caught N. vitripennis females are significantly more accepting of Sarcophaga hosts than are N. giraulti.
We next tested standard N. giraulti and N. vitripennis laboratory strains for host preference and acceptance. In preference tests, wherein wasps were provided with one Sarcophaga and one Protocalliphora host, N. giraulti showed a clear preference for Protocalliphora, whereas N. vitripennis showed a preference for Sarcophaga (see Figure 2). N. vitripennis was significantly more likely to both contact and sting Sarcophaga than Protocalliphora (contact: χ21=9.5, P<0.01; stinging: χ21=10.1, P<0.01), whereas N. giraulti had a significantly greater probability of contacting and stinging Protocalliphora hosts than Sarcophaga (contact: χ21=14.4, P<0.001; stinging: χ21=17.0, P<0.001). As can been seen in Figure 3 N. vitripennis also spent significantly more time on the Sarcophaga host than N. giraulti (Mann–Whitney U-test, z=8.0, P<0.0001), whereas N. giraulti spent significantly more time on the Protocalliphora host than N. vitripennis (Mann–Whitney U test, z=6.2, P<0.0001).
Behavior of wasps in host preference experiments. Nasonia vitripennis strains ASymCx and peach, Nasonia giraulti strain RV2Xu, and heterozygous bkbwg/+v introgression females were tested. Genetic content of the wasps is shown in the chromosomes to the right, with white representing Nasonia vitripennis DNA and black representing Nasonia giraulti DNA. As can be seen, heterozygous bkbwg/+v females contain a small region of Nasonia giraulti DNA in a largely Nasonia vitripennis genetic background. Wasps were given one Sarcophaga and one Protocalliphora host and observed for 4.5 h. The percent which contacted and stung each host is shown, and error bars indicate standard error of proportions (Sokal and Rohlf, 1969). Sample size for each strain is given to the right of the strain names.
Time spent on hosts by wasps in host acceptance and preference experiments. Nasonia vitripennis strains ASymCx and peach, Nasonia giraulti strain RV2Xu, and heterozygous bkbwg/+v introgression females were tested. Percent time is based on 28 observations over a 4.5 h period. Sample sizes for each strain in each experiment are shown to the right of the strain names. The relative sizes of Sarcophaga and Protocalliphora hosts are also shown.
However, when the laboratory strains of both species were presented with two Sarcophaga hosts in acceptance experiments, both strains were highly accepting of Sarcophaga (84%, N=89 for N. vitripennis and 78%, N=79 for N. giraulti). There was no significant difference between the two strains relative to whether they contacted or stung the Sarcophaga hosts (contact: χ21=0.20, P=0.65; stinging: χ21=0.66, P=0.42), although N. giraulti spent significantly less time on the Sarcophaga host than did N. vitripennis (Mann–Whitney U test, z=2.8, P<0.01; see Figure 3).
Introgression of the bkbwg region into N. vitripennis shows giraulti-like preference
Next, we tested host preference and acceptance of the bkbwg introgression strain. Preliminary tests of bkbwg/bkbwg homozygous females showed a complete failure to successfully parasitize Sarcophaga and Protocalliphora hosts in both the Sarcophaga and Protocalliphora host choice experiments and the two Sarcophaga acceptance experiments (data not shown). We therefore focused our host choice and acceptance experiments on bkbwg/+v heterozygous females, and as an additional control, we tested the preference of mutant N. vitripennis strain peach (the genetic background of the bkbwg introgression line).
The peach N. vitripennis strain shows the same host preferences as the standard N. vitripennis strain, as it was not significantly different from non-mutant N. vitripennis strain ASymCx for any behaviors (contact: χ21=0.64, P=0.42; stinging: χ21=1.3, P=0.25; see Figure 2). Peach was significantly different from N. giraulti for all behaviors (contact: χ21=21.8, P<0.001; stinging: χ21=23.2, P<0.001; host stung first: χ21=28.4, P<0.001). Therefore, it appears that the peach mutation (pe333) does not effect host preference in any significant way.
In contrast, heterozygous bkbwg/+v females showed a N. giraulti-like host preference in all behaviors (contact: χ21=0.31, P=0.58; stinging: χ21=0.49, P=0.48; see Figure 2). Heterozygous bkbwg/+v females were significantly more likely to contact and sting Protocalliphora than Sarcophaga in the 4.5 observation period (contact: χ21=13.1, P<0.001; stinging: χ21=27.6, P<0.001). Also, they spent similar amounts of time contacting each host as N. giraulti did (Mann–Whitney U test, time on Protocalliphora, z=0.9 P=0.18, time on Sarcophaga, z=0.7, P=0.25; see Figure 3). Heterozygous bkbwg/+v females show a N. giraulti-like preference for Protocalliphora in a N. vitripennis-like genetic background, suggesting that the N. giraulti preference for Protocalliphora is dominant. The trait segregates in a Mendelian manner.
In the two Sarcophaga acceptance experiments, bkbwg/+v heterozygous females did not show a significantly reduced contact rate relative to N. giraulti (χ21=0.45, P=0.42), but they did show a significantly reduced stinging rate (χ21=4.1, P<0.05). However, the stinging rate of the bkbwg/+v heterozygous females was still relatively high (65%) and they did not spend significantly less time contacting hosts than N. giraulti (Mann–Whitney U test, z=1.4, P=0.08; see Figure 3), suggesting that only minor genetic incompatibility effects occur in bkbwg/+v heterozygous females.
The bkbwg region maps to the centromeric portion of chromosome 4
We utilized the Nasonia genotyping microarray to genotype all scaffolds (contiguous DNA sequences) on chromosome 4 in a bkbwg introgression male (Table 1). For the majority of scaffolds, loci within a single scaffold were either scored as almost all N. vitripennis or almost all N. giraulti, allowing easy assignment of genotype to the scaffold as a whole. The exceptions to this were four scaffolds 23, 29, 40, 52, in which each contain a region scored mostly as N. vitripennis adjacent to a region scored mostly as N. giraulti. Scaffolds 23 and 40 represent the outer bounds of the introgressed region. A few additional scaffolds, namely 26 and 133, were scored mostly as N. giraulti, but with a small number of internal consecutive loci scored as having a N. vitripennis genotype.
The bkbwg region maps to the central portion of chromosome 4 (markers 4.18–4.25 in Niehuis et al., 2010), encompassing 13 complete and 4 incomplete major (>350 Kb) scaffolds totalling 11 Mb. The region also contains approximately 29 smaller scaffolds, bringing the total number of scaffolds to 46 and the total size of the introgressed region to approximately 16 Mb, and is a region of low recombination. Included within the region appears to be a 4.5 Mb stretch of N. vitripennis DNA (Table 1). Various lines of evidence indicate that the bkbwg region spans the centromere (Werren et al., 2010). Although large, this region contains a wealth of visible and molecular markers, which can be used to fine-scale map the host preference allele.
Discussion
Nasonia giraulti shows a clear preference for Protocalliphora, the host genus it parasitizes in nature, over Sarcophaga. N. vitripennis, known to be a generalist from field studies, shows a preference for Sarcophaga in choice experiments. The host preference behavior of N. giraulti was introgressed into the genome of its sibling species N. vitripennis, along with chromosomal regions linked to the bkbwg loci. Our genetic analysis indicates one or more genes linked to the bkbwg region strongly influence host preference and that this effect segregates in a Mendelian manner. Females heterozygous for the bkbwg region show strong preference for Protocalliphora hosts with only minimal signs of reduced vigor, suggesting that a host-preference effect is present in the region independent of any hybrid viability effects. The preference is all the more remarkable, given that Sarcophaga hosts are much larger than Protocalliphora hosts (see Figure 3 for relative sizes), and therefore would be more likely to be encountered in the experiment. To our knowledge, this is the first report of the introgression of host preference from one parasitoid species into another one.
The preference for Protocalliphora seen in bkbwg/+v heterozygous females also indicates the dominance of the N. giraulti allele. Based on the results, we posit a host preference locus (hp1) within this region with preference for Protocalliphora dominant over non-preference. A pattern of dominance in the inheritance of oviposition preference has been found in several phytophagous insects (for example, Huettel and Bush, 1972; Jaenike, 1987; Keese, 1996), although all of these examples represent specialist versus specialist comparisons rather than the specialist versus generalist comparison done here. Additive genetic variance for oviposition preference has also been reported (for example, Schneider and Roush, 1987; Sheck and Gould, 1995; Tucic et al., 1997; Messina and Slade, 1997). If specialization in Nasonia is derived, a dominant allele could have rapidly swept through a diverging population.
As the bkbwg/+v heterozygous females show host preference similar to N. giraulti, it is then possible that host preference in Nasonia is controlled by a small number of loci or clusters of tightly linked loci. A moderate number of loci have been found controlling host preference in other insects; (Jones, 2005) found an oligogenic basis (intermediate genetic complexity) to preference of Drosophila sechellia for a chemical attractant (Morinda fruit toxin) present in their preferred host plant. In addition, (Hawthorne and Via, 2001) found four separate quantitative trace loci affecting host plant choice in host races of the aphid Acyrthosiphon pisum. This is directly relevant to some speciation models in which speciation is more likely when there are fewer loci controlling host preference (Fry, 2003).
For the evolution of specialization, differential performance on hosts is an important element in addition to differential preference. Although field-caught N. giraulti showed highly reduced acceptance of Sarcophaga hosts, the laboratory strain RV2Xu did not. This is not unexpected, as N. giraulti does not appear to parasitize Sarcophaga in the wild, but the standard strain has been reared in the laboratory on Sarcophaga for several years (Protocalliphora cannot be reared in the laboratory). Therefore, it is possible that N. giraulti laboratory strains such as RV2Xu have developed an increased acceptance of Sarcophaga hosts. Clearly, however, this increased acceptance has not resulted in a preference for Sarcophaga over Protocalliphora in N. giraulti laboratory strain RV2Xu. This suggests that loci controlling preference and acceptance (that is, performance) may be unlinked, a requirement for some speciation models (Bush, 1975; Fry, 2003). However, a change in host acceptance could be because of genetic or environmental causes, and further studies are needed to determine the genetic basis of host acceptance differences in Nasonia species.
The results presented here indicate that a host preference gene is linked to the bkbwg locus, encompassed by 16 Mb of N. giraulti DNA around the centromere of chromosome 4. Within the introgressed bkbwg region appears to be a 4.5 Mb stretch of N. vitripennis DNA (Table 1). This may be the result of double recombination moving N. vitripennis DNA back into the introgressed region, possibly due to a gene in the region having a strong hybrid incompatibility effect. For example, the failure of bkbwg/bkbwg homozygous females to parasitize any tested hosts may be indicative of N. giraulti alleles linked to the larger bkbwg region that cause genetic incompatibilities when homozygous in an N. vitripennis genetic background. It is also possible that the bkbwg region is contiguous and the region in question actually belongs to a different region of the genome, but has been placed here by a combination of assembly and mapping errors. A few internal scaffolds also contained small stretches of N. vitripennis DNA. Given the low-recombination rate in the region, it is unlikely that these regions are the result of double recombination moving N. vitripennis DNA back into the bkbwg region and more likely that they are misassembled and actually belong in other parts of the genome, as only a single assembly error is required to explain each of these regions. It is now necessary to partition the region by recombination to produce more targeted introgressions of the host preference allele, and this work is underway. This is being accomplished using mapping resources available for Nasonia (Niehuis et al., 2010; Werren et al., 2010) and several visible markers present within the region (Figure 1).
The presence of a N. giraulti preference allele allows us to make some inferences on the evolution of host preference in Nasonia. It suggests that the transition between generalist and specialist strategies in Nasonia was not only an expansion or contraction of host range, but also included an actual change in preference for Protocalliphora hosts parasitized by both specialists and generalists. It is unknown whether either changes in host range or changes in host preference occurred first or whether they occurred simultaneously. Given that Trichomalopsis, close relatives of Nasonia, are largely generalists (Gibson and Floate, 2001), it is likely that generalism was the ancestral state for Nasonia, with subsequent evolution of specialization on Protocalliphora in the common ancestor of N. giraulti, N. longicornis and N. oneida.
The evolution of oviposition preference is considered to be one of the driving forces in the divergence of phytophagous insect populations (Futuyma, 1987; Thompson, 1993). Similar views have been presented for the divergence of parasitic Hymenoptera (Godfray, 1994). In parasitoids, the differential usage of hosts may produce assortative mating as a pleiotropic consequence of female oviposition behavior. As Nasonia species mate locally on patchily distributed hosts and routinely inbreed (Drapeau and Werren, 1999), host preference differences might quickly lead to assortative mating. It is interesting to note that N. giraulti shows a high propensity to mate within the host (Drapeau and Werren, 1999), producing a strong coupling of host preference and assortative mating. Therefore, it is possible that a shift in host preference was coupled with speciation events and perhaps with genetic bottlenecks because of the host shift. The low levels of genetic variation observed within Nasonia species is consistent with this scenario (Raychoudhury et al., 2010).
Conclusions
The results clearly indicate a major host preference locus (or set of tightly linked loci) in the region around bkbwg. The N. giraulti allele segregates in a Mendelian manner and imparts a dominant preference for Protocalliphora hosts in an otherwise N. vitripennis genetic background. Utilizing the Nasonia genotyping microarray, we have mapped the host preference effect to 16 Mb of chromosome 4. Fine-scale mapping of the host preference locus can now proceed, utilizing the wealth of mapping and molecular resources becoming available for Nasonia (Werren et al., 2010). To our knowledge, this is the first introgression of a host preference locus from one parasitoid species into another. Furthermore, this work represents one of the few examples of introgression of behavioral genes between species.
References
Breeuwer JAJ, Werren JH (1990). Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature 346: 558–560.
Breeuwer JAJ, Werren JH (1995). Hybrid breakdown between two haplodiploid species: the role of nuclear and cytoplasmic genes. Evolution 49: 705–717.
Bush GL (1975). Sympatric speciation in phytophagous parasitic insects. In: Price PW (ed). Evolutionary Strategies of Insects and Mites. Plenum Press: New York. pp 187–206.
Darling DC, Werren JH (1990). Biosystematics of two new species of Nasonia (Hymenoptera: Pteromalidae) reared from birds’ nests in North America. Ann Entomol Soc Am 83: 352–370.
Dworkin I, Jones CD (2009). Genetic changes accompanying the evolution of host specialization in Drosophila sechellia. Genetics 181: 721–736.
Dieckmann U, Doebelli M (1999). On the origin of species by sympatric speciation. Nature 400: 354–357.
Drapeau M, Werren JH (1999). Differences in mating behavior and sex ratio between three sibling species of Nasonia. Evol Ecol Res 1: 223–234.
Forbes AA, Powell THQ, Stelinski LL, Smith JJ, Feder JL (2009). Sequential sympatric speciation across trophic levels. Science 323: 776–779.
Fry JD (2003). Multilocus models of sympatric speciation: Bush versus Rice versus Felsenstein. Evolution 57: 1735–1746.
Futuyma DJ (1987). The role of behavior in host-associated divergence in herbivorous insects. In: Huettel MD (ed). Evolutionary Genetics of Invertebrate Behavior. Plenum Press: New York. pp 295–302.
Futuyma DJ, Mitter C (1996). Insect-plant interactions: the evolution of component communities. Philos Trans R Soc Lond B Biol Sci 351: 1361–1366.
Geervliet JBF, Vreugdenhill AI, Dicke M, Vet LEM (1998). Learning to discriminate between infochemicals from different plant-host complexes by the parasitoids Cotesia glomerata and C. rubecula. Entomol Exp Appl 86: 241–252.
Gibson GAP, Floate K (2001). Species of Trichomalopsis (Hymenoptera: Pteromalidae) associated with filth flies (Diptera: Muscidae) in North America. Can Entomol 133: 49–85.
Godfray HCJ (1994). Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press: Princeton: New Jersey.
Groman JD, Pellmyr O (2000). Rapid evolution and specialization following host colonization in a yucca moth. J Evol Biol 13: 223–236.
Hawthorne DJ, Via S (2001). Genetic linkage of ecological specialization and reproductive isolation in pea aphids. Nature 412: 904–907.
Huettel MD, Bush GL (1972). The genetics of host selection and its bearing on sympatric speciation in Procecidochares (Diptera: Tephritidae). Entomol Exp Appl 15: 465–480.
Jaenike J (1987). Genetics of oviposition-site preference in Drosophila tripunctata. Heredity 59: 363–369.
Jaenike J (1990). Host specialization in phytophagous insects. Annu Rev Ecol Syst 21: 243–273.
Jones CD (2005). The genetics of adaptation in Drosophila sechellia. Genetica 123: 137–145.
Kawecki TJ (1998). Red Queen meets Santa Rosalia: arms races and the evolution of host specialization in organisms with parasitic lifestyles. Am Nat 152: 635–651.
Keese MC (1996). Feeding responses of hybrids and the inheritance of host-use traits in leaf feeding beetles (Coleoptera: Chrysomelidae). Heredity 76: 36–42.
Kelley ST, Farrell BD (1998). Is specialization a dead end? The phylogeny of host use in dendroctonus bark beetles (Scolytidae). Evolution 52: 1731–1743.
Knowles LL, Levy A, McNellis JM, Greene KP, Futuyma DJ (1999). Tests of inbreeding effects on host-shift potential in the phytophagous beetle Ophraella communa. Evolution 53: 561–567.
Kondrashov AS, Kondrashov FA (1999). Interactions among quantitative traits in the course of sympatric speciation. Nature 400: 351–354.
Loehlin DW, Enders LS, Werren JH (2010). Evolution of sex-specific wing shape at the widerwing locus in four species of Nasonia. Heredity (doi:10.1038/hdy.2009.146).
Louis J, David JR (1986). Ecological specialization in the Drosophila melanogaster species subgroup: a case study of D sechellia. Acta Oecologica 7: 215–229.
Lynch JA, Desplan C (2006). A method for parental RNA interference in the wasp Nasonia vitripennis. Nat Protoc 1: 486–494.
Magalhães S, Forbes MR, Skoracka A, Osakabe M, Chevillon C, McCoy KD (2007). Host race formation in the Acari. Exp Appl Acarol 42: 225–238.
Matsuo T, Sugaya S, Yasukawa J, Aigaki T, Fuyama Y (2007). Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol 5: 985–996.
Messina FJ, Slade AF (1997). Inheritance of host-plant choice in the seed beetle Callosobruchus maculatus (Coleoptera: Bruchidae). Ann Entomol Soc Am 90: 848–855.
Niehuis O, Gibson JD, Rosenberg MS, Pannebakker BA, Koevoets T, Judson AK et al. (2010). Recombination and its impact of the genome of the haplodiploid parasitoid wasp Nasonia. PLoS One (doi:10.1371/journal.pone.008597).
Nylin S, Nygren GH, Windig JJ, Janz N, Bergstrom A (2005). Genetics of host-plant preference in the comma butterfly Polygonia c-album (Nymphalidae), and evolutionary implications. Biol J Linn Soc Lond 84: 755–765.
Quicke DL (1997). Parasitic WaspsAQ6. Chapman and Hall: London, UK.
Pannebakker B, Niehuis O, Hedley AA, Gadau J, Shuker DM (2010). The distribution of microsatellites in the Nasonia parasitoid wasp genome. Insect Mol Biol 19 (S1): 91–98.
Raychoudhury R, Desjardins CA, Buellesbach J, Loehlin DW, Grillenberger BK, Beukeboom L et al. (2010). Behavioural and genetic characteristics of a new species of Nasonia. Heredity (doi:10.1038/hdy.2009.147).
Rice WR (1987). Selection via habitat specialization, the evolution of reproductive isolation as a correlated character. Evol Ecol 1: 301–314.
Saul II GB, Kayhart M (1956). Mutants and linkage in Mormoniella. Genetics 41: 930–937.
Saul II GB, Whiting PW, Saul SW, Heidner CA (1965). Wild-type and mutant stocks of Mormoniella (Nasonia). Genetics 52: 1317–1327.
Saul II GB, Saul SW, Becker S (1967). Linkage in Mormoniella. Genetics 57: 369–384.
Schneider JC, Roush RT (1987). Genetic differences in oviposition preference between two populations of Heliothis virescens. In: Huettel MD (ed). Evolutionary Genetics of Invertebrate Behavior. Plenum Press: New York. pp 163–171.
Sheck AL, Gould F (1995). Genetic analysis of differences in oviposition preferences of Heliothis virescens and H. subflexa (Lepidoptera: Noctuidae). Environ Entomol 24: 341–347.
Sokal RR, Rohlf FJ (1969). Biometry 1st edn. W H Freeman and Company: San Francisco.
Thompson JN, Wehling W, Podolsky R (1990). Evolutionary genetics of host use in swallowtail butterflies. Nature 344: 148–150.
Thompson JN (1993). Preference hierarchies and the origin of geographic specialization in host use in swallowtail butterflies. Evolution 47: 1585–1594.
Tucic N, Mikuljanac S, Stojkovic O (1997). Genetic variation and covariation among life history traits in populations of Acanthoscelides obtectus maintained on different hosts. Entomol Exp Appl 85: 247–256.
Vinson SB (1998). The general host selction behavior of parasitoid hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Control 11: 79–96.
Werren JH, Richards S, Desjardins CA, Niehuis O, Gadau J, Colbourne JK et al. (2010). Functional evolutionary insights from the genome of three parasitoid Nasonia species. Science 327: 343.
Werren JH, Loehlin DW (2009). The parasitoid wasp Nasonia: an emerging model system with haploid male genetics. Cold Spring Harb Protoc (doi:10.1101/pdb.emo134).
Weston RF, Qureshi I, Werren JH (1999). Genetics of wing size differences between two Nasonia species. J Evol Biol 12: 586–595.
Whiting AR (1967). The biology of the parasitic wasp Mormoniella vitripennis [=Nasonia brevicornis] (Walker). Q Rev Biol 42: 233–406.
Whitlock MC (1996). The Red Queen beats the jack-of-all-trades: The limitations on the evolution of phenotypic plasticity and niche breadth. Am Nat 148: S65–S77.
Acknowledgements
We thank R Edwards, C Kennedy and S Patel for Nasonia maintenance; M Drapeau and S Bordenstein for assistance in data collection; A Avery and J Traggis for mapping visible markers in the bkbwg region; J Colbourne and J Lopez for conducting the mapping array hybridization experiments; M Clark, D Loehlin, J Jaenike, and three anonymous reviewers for helpful comments on the manuscript; and members of the North American Bluebird Society, particularly P Conklin, J Rogers and R Wells, for providing assistance in field work. Support for this research was provided by a postdoctoral fellowship from the Spanish Ministry of Education and Culture to FP and by the National Science Foundation (IBN-9876356) and National Institutes of Health (5R01GM070026) to JHW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desjardins, C., Perfectti, F., Bartos, J. et al. The genetic basis of interspecies host preference differences in the model parasitoid Nasonia. Heredity 104, 270–277 (2010). https://doi.org/10.1038/hdy.2009.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2009.145
Keywords
This article is cited by
-
Cavity-breeding birds create specific microhabitats for diverse arthropod communities in boreal forests
Biodiversity and Conservation (2023)
-
Genomics of sex allocation in the parasitoid wasp Nasonia vitripennis
BMC Genomics (2020)
-
Metamorphosis-related changes in the free fatty acid profiles of Sarcophaga (Liopygia) argyrostoma (Robineau-Desvoidy, 1830)
Scientific Reports (2020)
-
Variation in parasitoidism of Protocalliphora azurea (Diptera: Calliphoridae) by Nasonia vitripennis (Hymenoptera: Pteromalidae) in Spain
Parasitology Research (2020)
-
Genetic architecture underlying host choice differentiation in the sympatric host races of Lochmaea capreae leaf beetles
Genetica (2016)