Abstract
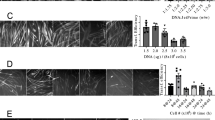
Modification of K+ currents by exogenous gene expression may lead to therapeutic interventions in skeletal muscle diseases characterized by alterations in electrical excitability. In order to study the specific effects of increasing outward K+ currents, we expressed a modified voltage-dependent K+ channel in primary cultured rat skeletal muscle cells. The rat Kv1.4 channel was expressed as an N-terminal fusion protein containing a bioluminescent marker (green fluorescent protein). Transgene expression was carried out using the helper-dependent herpes simplex 1 amplicon system. Transduced myoballs, identified using fluorescein optics and studied electrophysiologically with single-cell patch clamp, exhibited a greater than two-fold increase in K+ conductance by 20–30 h after infection. This increase in K+ current led to a decrease in membrane resistance and a 10-fold increase in the current threshold for action potential generation. Electrical hyperexcitability induced by the Na+ channel toxin anemone toxin II (1 μM) was effectively counteracted by overexpression of Kv1.4 at 30–32 h after transduction. Thus, virally induced overexpression of a voltage-gated K+ channel in skeletal muscle has a powerful effect in reducing electrical excitability. Gene Therapy (2001) 8, 1372–1379.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hille B . Ionic Channels of Excitable Membranes. Sinauer Assoc. Inc : Sunderland, MA 1992
Simonato M, Manservigi R, Marconi P, Glorioso JC . Gene transfer into neurons for the molecular analysis of behaviour: focus on herpes simplex vectors Trends Neurosci 2000 24: 183–190
Feero WG et al. Viral gene delivery to skeletal muscle: insights on maturation-dependent loss of fiber infectivity for adenovirus and herpes simplex type 1 viral vectors Hum Gene Ther 1997 8: 371–380
Glorioso JC, DeLuca NA, Fink DJ . Development and application of herpes simplex virus vectors for human gene therapy Annu Rev Microbiol 1995 49: 675–710
Huard J et al. Gene transfer to muscle using herpes simplex virus-based vectors Neuromusc Disord 1997 7: 299–313
Wang Y et al. HSV-1 amplicon vectors are a highly efficient gene delivery system for skeletal muscle myoblasts and myotubes Am J Physiol Cell Physiol 2000 278: C619–C626
Geller AI, Breakefield XO . A defective HSV-1 vector expresses Escherichia coli β-galactosidase in cultured peripheral neurons Science 1988 241: 1667–1669
Spaete RR, Frenkel N . The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector Cell 1982 30: 295–304
Burke NA et al. Distinct structural requirements for clustering and immobilization of K+ channels by PSD-95 J Gen Physiol 1999 113: 71–80
Hoshi T, Zagotta WN, Aldrich RW . Biophysical and molecular mechanisms of Shaker potassium channel inactivation Science 1990 250: 553–538
Falk T et al. A herpes simplex viral vector expressing green fluorescent protein can be used to visualize morphological changes in high-density neuronal culture E J Biotech [online] 2001 Available from:http://ejb.ucv.cl/content/vol4/issue1/full/5/index.html
Cannon SC . Sodium channel defects in myotonia and periodic paralysis Ann Rev Neurosci 1996 19: 141–164
Vullhorst D, Klocke R, Bartsch JW, Jokusch H . Expression of the potassium channel Kv3.4 in mouse skeletal muscle parallels fiber type maturation and depends on excitation pattern FEBS Lett 1998 421: 259–262
Weiser M et al. Differential expression of Shaw-related K+ channels in the rat central nervous system J Neurosci 1994 14: 949–972
Vigdor-Alboim S et al. Discoordinate regulation of different K channels in cultured rat skeletal muscle by nerve growth factor J Neurosci Res 1999 56: 275–283
Kalman K et al. Genomic organization, chromosomal localization, tissue distribution, and biophysical characterization of a novel mammalian Shaker-related voltage-gated potassium channel, Kv1.7 J Biol Chem 1998 273: 5851–5857
Sharma N et al. Nerve growth factor regulates the abundance and distribution of K+ channels in PC12 cells J Cell Biol 1993 123: 1835–1843
Roberds SL, Tamkun MM . Cloning and tissue-specific expression of five voltage-gated potassium channel cDNAs expressed in rat heart Proc Natl Acad Sci USA 1991 88: 1798–1802
Catterall WA . From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels Neuron 2000 26: 13–25
Lehmann-Horn F, Jurkatt-Roth K . Voltage-gated ion channels and hereditary disease Physiol Rev 1999 79: 1317–1372
Sambrook J, Fritsch EF, Maniatis T . Molecular Cloning: A Laboratory Handbook Cold Spring Harbor Press: Cold Spring Harbor, NY 1989
Prasher DC et al. Primary structure of the Aequoria victoria green-fluorescent protein Gene 1992 111: 229–233
Dyer AP, Tufaro F . Herpes simplex virus vector for gene therapy of the nervous system. In: Fodoroff S, Richardson A (eds) Protocols for Neural Cell Culture Humana Press: Totowa, NJ 1997 185–196
Gonoi T, Sherman SJ, Catterall WA . Voltage clamp analysis of tetrodotoxin-sensitive and -insensitive sodium channels in rat muscle cells developing in culture J Neurosci 1985 5: 2559–2564
Acknowledgements
This work was supported by the Muscular Dystrophy Association (AJY), NINDS NS202015–03 and the Nielsen Trust (SJS) and NIH 1 RO1 MH59747-O1A1 (AJY). We thank Lori Strazdas and Amy H Marble for expert technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Falk, T., Kilani, R., Yool, A. et al. Viral vector-mediated expression of K+ channels regulates electrical excitability in skeletal muscle. Gene Ther 8, 1372–1379 (2001). https://doi.org/10.1038/sj.gt.3301539
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301539