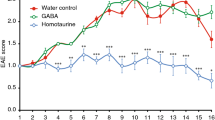
Abstract
The peripheral delivery of drugs in patients affected by central nervous system (CNS)-confined diseases is therapeutically ineffective due to the presence of the blood–brain barrier which forms an inaccessible wall to the majority of CNS targeting molecules. When molecules with an anti-inflammatory profile have been systemically administered to patients affected by a chronic inflammatory demyelinating disease of the CNS, such as multiple sclerosis (MS), results have been disappointing. A successful therapeutic approach in MS should therefore consider the delivery of anti-inflammatory molecules directly into the CNS in order to inhibit blood-borne CNS-confined mononuclear cells which act as ultimate effector cells directly destroying oligodendrocytes and/or releasing myelinotoxic substances. Biological and physical vectors engineered with heterologous genes coding for immunomodulatory cytokines with an anti-inflammatory profile might represent the appropriate tool to deliver therapeutic genes into the CNS of patients with MS. So far, cytokine gene therapy has never been attempted in MS, but encouraging results have been obtained in the animal model of MS, experimental autoimmune encephalomyelitis (EAE), using viral vectors or plasmids engineered with cytokine genes and then injected systemically, either in the blood stream or circulating encephalitogenic T cells, or into the CNS. Here, we critically discuss the various attempts made in EAE using gene therapy protocols based on the delivery of immunomodulatory cytokine genes. Special emphasis is put on the use of non-replicative herpes simplex type-1 (HSV)-derived vectors engineered with the gene of the immunomodulatory cytokine interleukin (IL)-4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Steinman L . A few autoreactive cells in an autoimmune infiltrate control a vast population of nonspecific cells: a tale of smart bombs and the infantry Proc Natl Acad Sci USA 1996 93: 2253–2256
Martino G, Hartung HP . Immunopathogenesis of multiple sclerosis: the role of T cells Curr Opin Neurol 1999 12: 309–321
Kieseier B et al. Effector pathways in immune mediated central nervous system demyelination Curr Opin Neurol 1999 12: 323–336
Abbas AK, Murphy KM, Sher A . Functional diversity of helper T lymphocytes Nature 1996 383: 787–793
Mosmann TR, Sad S . The expanding universe of T-cell subsets: Th1, Th2 and more Immunol Today 1996 17: 138–146
Panitch NL, Hirsch RL, Haley AS, Johnson KP . Exacerbations of multiple sclerosis in patients treated with gamma interferon Lancet 1987 1: 893–895
Panitch NL, Hirsch RL, Schindler J, Johnson KP . Treatment of MS with gamma interferon: exacerbations associated with activation of the immune system Neurology 1987 37: 1097–1102
Selmaj KW, Raine CS, Cannella B, Brosnan CF . Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions J Clin Invest 1991 87: 949–954
Beck J et al. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand 1988 78: 318–323
Calabresi PA, Tranquill LR, McFarland HF, Cowan EP . Cytokine gene expression in cells derived from CSF of multiple sclerosis patients J Neuroimmunol 1998 89: 198–205
Martino G et al. Proinflammatory cytokines regulate antigen-independent T cell activation by two separate calcium signaling pathways in multiple sclerosis patients Ann Neurol 1998 43: 340–349
Correale J et al. Patterns of cytokine secretion by autoreactive proteolipid protein-specific T cell clones during the course of multiple sclerosis J Immunol 1995 154: 2959–2968
Sørensen TL et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis J Clin Invest 1999 103: 6807–6815
Olsson T . Cytokine-producing cells in experimental autoimmune encephalomyelitis and multiple sclerosis Neurology 1995 45 (Suppl. 6): S11–S15
Furlan R et al. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination J Immunol 1999 163: 2403–2409
Meinl E et al. Encephalitogenic potential of myelin basic protein-specific T cells isolated from normal rhesus macaques Am J Pathol 1997 150: 445–453
The IFNB Multiple Sclerosis Study Group . Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial Neurology 1993 43: 655–661
Weinstock-Guttman B, Ransohoff RM, Kinkel RP, Rudick RA . The interferons: biological effects, mechanisms of action, and use in multiple sclerosis Ann Neurol 1995 37: 7–15
Calabresi PA et al. Phase 1 trial of transforming growth factor beta 2 in chronic progressive MS Neurology 1998 51: 289–292
The Lenercept Multiple Sclerosis Study Group and University of British Columbia MS/MRI Analysis Group . TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study Neurology 1999 53: 457–465
Saunders NR, Habgood MD, Dziegielewska KM . Barrier mechanisms in the brain, I. Adult brain Clin Exp Pharmacol Physiol 1999 26: 11–19
Khan OA et al. Interferon beta-1b serum levels in multiple sclerosis patients following subcutaneous administration Neurology 1996 46: 1639–1643
The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group . Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years Neurology 1996 47: 889–894
Shaw MK et al. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis J Exp Med 1997 185: 1711–1174
Dal Canto RA et al. Local delivery of TNF by retrovirus-transduced T lymphocytes exacerbates experimental autoimmune encephalomyelitis Clin Immunol 1999 90: 10–14
Chen LZ et al. Gene therapy in allergic encephalomyelitis using myelin basic protein-specific T cells engineered to express latent transforming growth factor-betal Proc Natl Acad Sci USA 1998 95: 12516–12521
Mathisen PM et al. Treatment of experimental autoimmune encephalomyelitis with genetically modified memory T cells J Exp Med 1997 186: 159–164
Willenborg DO, Fordham SA, Cowden WB, Ramshaw IA . Cytokines and murine autoimmune encephalomyelitis: inhibition or enhancement of disease with antibodies to select cytokines, or by delivery of exogenous cytokines using a recombinant vaccinia virus system Scand J Immunol 1995 41: 31–40
Croxford JL et al. Cytokine gene therapy in experimental allergic encephalomyelitis by injection of plasmid DNA-cationic liposome complex into the central nervous system J Immunol 1997 160: 5181–5187
Triantaphyllopoulos K, Croxford J, Baker D, Chernajovsky Y . Cloning and expression of murine IFN beta and a TNF antagonist for gene therapy of experimental allergic encephalomyelitis Gene Therapy 1998 5: 253–263
Piccirillo CA, Prud'homme GJ . Prevention of experimental allergic encephalomyelitis by intramuscular gene transfer with cytokine-encoding plasmid vectors Hum Gene Ther 1999 10: 1915–1922
Boccaccio GL, Mor F, Steinman L . Non-coding plasmid DNA induces IFN-gamma in vivo and suppresses autoimmune encephalomyelitis Intern Immunol 1999 11: 289–296
Tsunoda I et al. Exacerbation of viral and autoimmune animal models for multiple sclerosis by bacterial DNA Brain Pathol 1999 9: 481–493
Glorioso JC et al. Gene transfer to brain using herpes simplex virus vectors Ann Neurol 1994 35: S28–34
Krisky DM et al. Rapid method for construction of recombinant HSV-1 gene transfer vectors Gene Therapy 1997 4: 1120–1125
Marconi P et al. Replication-defective herpes simplex virus vectors for gene transfer in vivo Proc Natl Acad Sci USA 1996 93: 11319–11320
DeLuca NA, McCarthy AM, Schaffer PA . Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate–early regulatory protein ICP4 J Virol 1985 56: 558–570
Kuklin NA et al. Modulation of mucosal and systemic immunity by enteric administration of non-replicating herpes simplex virus expressing cytokines Virology 1998 240: 245–253
Martino G et al. A gene therapy approach to treat demyelinating diseases using non-replicative herpetic vectors engineered to produce cytokines Mult Scler 1998 4: 222–227
Furlan R et al. Central nervous system delivery of interleukin-4 by a non-replicative herpes simplex type 1 viral vector ameliorates autoimmune demyelination Hum Gene Ther 1998 9: 2605–2617
Furlan R et al. Interleukin-4 gene delivery in the central nervous inhibits ongoing experimental autoimmune encephalomyelitis (submitted for publication)
Acknowledgements
Our work is supported by Telethon, Minister of Health (Progetti Finalizati) and Italian National Multiple Sclerosis Society (AISM). We thank Joseph Glorioso for providing the HSV-1-derived vectors and encouraging our research.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martino, G., Poliani, P., Marconi, P. et al. Cytokine gene therapy of autoimmune demyelination revisited using herpes simplex virus type-1-derived vectors. Gene Ther 7, 1087–1093 (2000). https://doi.org/10.1038/sj.gt.3301215
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301215
Keywords
This article is cited by
-
Expression of interleukin-4 but not of interleukin-10 from a replicative herpes simplex virus type 1 viral vector precludes experimental allergic encephalomyelitis
Gene Therapy (2001)
-
Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics
Nature Medicine (2001)
-
Central nervous system gene therapy with interleukin-4 inhibits progression of ongoing relapsing–remitting autoimmune encephalomyelitis in Biozzi AB/H mice
Gene Therapy (2001)
-
Fibroblast growth factor-II gene therapy reverts the clinical course and the pathological signs of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice
Gene Therapy (2001)