Abstract
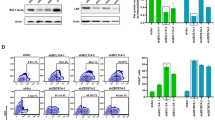
Correction of patient-specific induced pluripotent stem cells (iPSC) upon gene delivery through retroviral vectors offers new treatment perspectives for monogenetic diseases. Gene-modified iPSC clones can be screened for safe integration sites and differentiated into transplantable cells of interest. However, the current bottleneck is epigenetic vector silencing. In order to identify the most suitable retroviral expression system in iPSC, we systematically compared vectors from different retroviral genera, different promoters and their combination with ubiquitous chromatin opening elements (UCOE), and several envelope pseudotypes. Lentiviral vectors (LV) pseudotyped with vesicular stomatitis virus glycoprotein were superior to gammaretroviral and alpharetroviral vectors and other envelopes tested. The elongation factor 1α short (EFS) promoter mediated the most robust expression, whereas expression levels were lower from the potent but more silencing-prone spleen focus forming virus (SFFV) promoter. Both full-length (A2UCOE) and minimal (CBX3) UCOE juxtaposed to two physiological and one viral promoter reduced transgene silencing with equal efficiency. However, a promoter-specific decline in expression levels was not entirely prevented. Upon differentiation of transgene-positive iPSC into endothelial cells, A2UCOE.EFS and CBX3.EFS vectors maintained highest transgene expression in a larger fraction of cells as compared with all other constructs tested here. The function of UCOE diminished, but did not fully counteract, vector silencing and possibilities for improvements remain. Nevertheless, the CBX3.EFS in a LV background exhibited the most promising promoter and vector configuration for both high titer production and long-term genetic modification of human iPSC and their progeny.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schambach A, Cantz T, Baum C, Cathomen T . Generation and genetic modification of induced pluripotent stem cells. Expert Opin Biol Ther 2010; 10: 1089–1103.
Cherry AB, Daley GQ . Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med 2013; 64: 277–290.
Ellis J . Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther 2005; 16: 1241–1246.
Mok HP, Javed S, Lever A . Stable gene expression occurs from a minority of integrated HIV-1-based vectors: transcriptional silencing is present in the majority. Gene Ther 2007; 14: 741–751.
Minoguchi S, Iba H . Instability of retroviral DNA methylation in embryonic stem cells. Stem Cells 2008; 26: 1166–1173.
Herbst F, Ball CR, Tuorto F, Nowrouzi A, Wang W, Zavidij O et al. Extensive methylation of promoter sequences silences lentiviral transgene expression during stem cell differentiation in vivo. Mol Ther 2012; 20: 1014–1021.
Wolf D, Goff SP . TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 2007; 131: 46–57.
Schlesinger S, Lee AH, Wang GZ, Green L, Goff SP . Proviral silencing in embryonic cells is regulated by Yin Yang 1. Cell Rep 2013; 4: 50–58.
Wolf D, Goff SP . Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 2009; 458: 1201–1204.
Grez M, Akgun E, Hilberg F, Ostertag W . Embryonic stem cell virus, a recombinant murine retrovirus with expression in embryonic stem cells. Proc Natl Acad Sci USA 1990; 87: 9202–9206.
Ramezani A, Hawley TS, Hawley RG . Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol Ther 2000; 2: 458–469.
Ramezani A, Hawley TS, Hawley RG . Stable gammaretroviral vector expression during embryonic stem cell-derived in vitro hematopoietic development. Mol Ther 2006; 14: 245–254.
Antoniou MN, Skipper KA, Anakok O . Optimizing retroviral gene expression for effective therapies. Hum Gene Ther 2013; 24: 363–374.
Emery DW . The use of chromatin insulators to improve the expression and safety of integrating gene transfer vectors. Hum Gene Ther 2011; 22: 761–774.
Antoniou M, Harland L, Mustoe T, Williams S, Holdstock J, Yague E et al. Transgenes encompassing dual-promoter CpG islands from the human TBP and HNRPA2B1 loci are resistant to heterochromatin-mediated silencing. Genomics 2003; 82: 269–279.
Urbinati F, Arumugam P, Higashimoto T, Perumbeti A, Mitts K, Xia P et al. Mechanism of reduction in titers from lentivirus vectors carrying large inserts in the 3'LTR. Mol Ther 2009; 17: 1527–1536.
Puthenveetil G, Scholes J, Carbonell D, Qureshi N, Xia P, Zeng L et al. Successful correction of the human beta-thalassemia major phenotype using a lentiviral vector. Blood 2004; 104: 3445–3453.
Sharma N, Hollensen AK, Bak RO, Staunstrup NH, Schroder LD, Mikkelsen JG . The impact of cHS4 insulators on DNA transposon vector mobilization and silencing in retinal pigment epithelium cells. PloS One 2012; 7: e48421.
Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Schambach A, Sinclair J et al. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood 2007; 110: 1448–1457.
Zhang F, Frost AR, Blundell MP, Bales O, Antoniou MN, Thrasher AJ . A ubiquitous chromatin opening element (UCOE) confers resistance to DNA methylation-mediated silencing of lentiviral vectors. Mol Ther 2010; 18: 1640–1649.
Pfaff N, Lachmann N, Ackermann M, Kohlscheen S, Brendel C, Maetzig T et al. A ubiquitous chromatin opening element prevents transgene silencing in pluripotent stem cells and their differentiated progeny. Stem Cells 2013; 31: 488–499.
Ackermann M, Lachmann N, Hartung S, Eggenschwiler R, Pfaff N, Happle C et al. Promoter and lineage independent anti-silencing activity of the A2 ubiquitous chromatin opening element for optimized human pluripotent stem cell-based gene therapy. Biomaterials 2014; 35: 1531–1542.
Muller-Kuller U, Ackermann M, Kolodziej S, Brendel C, Fritsch J, Lachmann N et al. A minimal ubiquitous chromatin opening element (UCOE) effectively prevents silencing of juxtaposed heterologous promoters by epigenetic remodeling in multipotent and pluripotent stem cells. Nucleic Acids Res 2015; 43: 1577–1592.
Warlich E, Kuehle J, Cantz T, Brugman MH, Maetzig T, Galla M et al. Lentiviral vector design and imaging approaches to visualize the early stages of cellular reprogramming. Mol Ther 2011; 19: 782–789.
Voelkel C, Galla M, Maetzig T, Warlich E, Kuehle J, Zychlinski D et al. Protein transduction from retroviral Gag precursors. Proc Natl Acad USA 2010; 107: 7805–7810.
Kaufmann KB, Brendel C, Suerth JD, Mueller-Kuller U, Chen-Wichmann L, Schwable J et al. Alpharetroviral vector-mediated gene therapy for X-CGD: functional correction and lack of aberrant splicing. Mol Ther 2013; 21: 648–661.
Suerth JD, Morgan MA, Kloess S, Heckl D, Neudorfl C, Falk CS et al. Efficient generation of gene-modified human natural killer cells via alpharetroviral vectors. J Mol Med 2016; 94: 83–93.
Labenski V, Suerth JD, Barczak E, Heckl D, Levy C, Bernadin O et al. Alpharetroviral self-inactivating vectors produced by a superinfection-resistant stable packaging cell line allow genetic modification of primary human T lymphocytes. Biomaterials 2016; 97: 97–109.
Suerth JD, Maetzig T, Brugman MH, Heinz N, Appelt JU, Kaufmann KB et al. Alpharetroviral self-inactivating vectors: long-term transgene expression in murine hematopoietic cells and low genotoxicity. Mol Ther 2012; 20: 1022–1032.
Hacein-Bey-Abina S, Pai SY, Gaspar HB, Armant M, Berry CC, Blanche S et al. A modified gamma-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med 2014; 371: 1407–1417.
Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 2013; 341: 1233151.
Naldini L . Gene therapy returns to centre stage. Nature 2015; 526: 351–360.
Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 2009; 460: 53–59.
Lachmann N, Happle C, Ackermann M, Luttge D, Wetzke M, Merkert S et al. Gene correction of human induced pluripotent stem cells repairs the cellular phenotype in pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2014; 189: 167–182.
Papapetrou EP, Lee G, Malani N, Setty M, Riviere I, Tirunagari LM et al. Genomic safe harbors permit high beta-globin transgene expression in thalassemia induced pluripotent stem cells. Nat Biotechnol 2010; 29: 73–78.
Morishima T, Watanabe K, Niwa A, Hirai H, Saida S, Tanaka T et al. Genetic correction of HAX1 in induced pluripotent stem cells from a patient with severe congenital neutropenia improves defective granulopoiesis. Haematologica 2014; 99: 19–27.
Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M . LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci USA 2013; 110: 7306–7311.
Amirache F, Levy C, Costa C, Mangeot PE, Torbett BE, Wang CX et al. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood 2014; 123: 1422–1424.
Rasko JE, Battini JL, Gottschalk RJ, Mazo I, Miller AD . The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc Natl Acad Sci USA 1999; 96: 2129–2134.
Norrman K, Fischer Y, Bonnamy B, Wolfhagen Sand F, Ravassard P, Semb H . Quantitative comparison of constitutive promoters in human ES cells. PloS One 2010; 5: e12413.
Rival-Gervier S, Lo MY, Khattak S, Pasceri P, Lorincz MC, Ellis J . Kinetics and epigenetics of retroviral silencing in mouse embryonic stem cells defined by deletion of the D4Z4 element. Mol Ther 2013; 21: 1536–1550.
Papapetrou EP, Schambach A . Gene insertion into genomic safe harbors for human gene therapy. Mol Ther 2016; 24: 678–684.
Ordovas L, Boon R, Pistoni M, Chen Y, Wolfs E, Guo W et al. Efficient recombinase-mediated cassette exchange in hPSCs to study the hepatocyte lineage reveals AAVS1 locus-mediated transgene inhibition. Stem Cell Rep 2015; 5: 918–931.
Kuehle J, Turan S, Cantz T, Hoffmann D, Suerth JD, Maetzig T et al. Modified lentiviral LTRs allow Flp recombinase-mediated cassette exchange and in vivo tracing of "Factor-free" induced pluripotent stem cells. Mol Ther 2014; 22: 919–928.
Suerth JD, Maetzig T, Galla M, Baum C, Schambach A . Self-inactivating alpharetroviral vectors with a split-packaging design. J Virol 2010; 84: 6626–6635.
Schambach A, Bohne J, Chandra S, Will E, Margison GP, Williams DA et al. Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. Mol Ther 2006; 13: 391–400.
Schambach A, Mueller D, Galla M, Verstegen MM, Wagemaker G, Loew R et al. Overcoming promoter competition in packaging cells improves production of self-inactivating retroviral vectors. Gene Ther 2006; 13: 1524–1533.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Schott JW, Hoffmann D, Maetzig T, Muller FJ, Steinemann D, Zychlinski D et al. Improved retroviral episome transfer of transcription factors enables sustained cell fate modification. Gene Ther 2014; 21: 938–949.
Yee JK, Friedmann T, Burns JC . Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol 1994; 43 : 99–112.
Cone RD, Mulligan RC . High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci USA 1984; 81: 6349–6353.
Sandrin V, Boson B, Salmon P, Gay W, Negre D, Le Grand R et al. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood 2002; 100: 823–832.
Orlova VV, Drabsch Y, Freund C, Petrus-Reurer S, van den Hil FE, Muenthaisong S et al. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler Thromb Vasc Biol 2014; 34: 177–186.
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB738, Cluster of Excellence REBIRTH (EXC 62/1) and Mo886/6-1), the Bundesministerium für Bildung und Forschung (BMBF, Joint Research Project IFB-Tx, pid.net), the Deutscher Akademischer Austauschdienst (DAAD) and the European Union (FP7 projects PERSIST and CELLPID). FJM was supported by grants from the BMBF (13GW0128A and 01GM1513D) and from the Deutsche Forschungsgemeinschaft (German Research Foundation DFG MU 3231/3-1). We thank Johannes Kühle for providing viral supernatants, Gerald Draeger and Thomas Scheper for providing Rock inhibitor Y-27632, SB431542 inhibitor and bFGF (Leibnitz University, Hannover, Germany) as well as Malte Sgodda and Tobias Cantz for H9 ESC RNA (Hannover Medical School, Hannover, Germany). We would also like to thank Manuel Grez for his kind advice and support of this project and Adrian Schwarzer for his advice in statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Hoffmann, D., Schott, J., Geis, F. et al. Detailed comparison of retroviral vectors and promoter configurations for stable and high transgene expression in human induced pluripotent stem cells. Gene Ther 24, 298–307 (2017). https://doi.org/10.1038/gt.2017.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.20
This article is cited by
-
Inflammation-inducible promoters to overexpress immune inhibitory factors by MSCs
Stem Cell Research & Therapy (2023)
-
Site-specific transgene integration in chimeric antigen receptor (CAR) T cell therapies
Biomarker Research (2023)
-
Viruses as tools in gene therapy, vaccine development, and cancer treatment
Archives of Virology (2022)
-
Luminescent Human iPSC-Derived Neurospheroids Enable Modeling of Neurotoxicity After Oxygen–glucose Deprivation
Neurotherapeutics (2022)
-
Lentiviral gene therapy and vitamin B3 treatment enable granulocytic differentiation of G6PC3-deficient induced pluripotent stem cells
Gene Therapy (2020)