Abstract
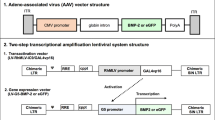
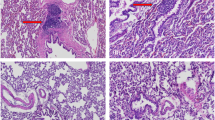
We have previously obtained compelling proof-of-principle evidence for COX2 gene therapy for fracture repair using integrating retroviral vectors. For this therapy to be suitable for patient uses, a suitable vector with high safety profile must be used. Accordingly, this study sought to evaluate the feasibility of AAV as the vector for this COX2 gene therapy, because AAV raises less safety issues than the retroviral vectors used previously. However, an appropriate AAV serotype is required to provide early increase in and adequate level of COX2 expression that is needed for fracture repair. Herein, we reported that AAV-DJ, an artificial AAV pseudoserotype, is highly effective in delivering COX2 gene to fracture sites in a mouse femoral fracture model. Compared with AAV-2, the use of AAV-DJ led to ~5-fold increase in infectivity in mesenchymal stem cells (MSCs) and provided an earlier and significantly higher level of transgene expression at the fracture site. Injection of this vector at a dose of 7.5 × 1011 genomic copies led to high COX2 level at the fracture site on day 3 after injections and significantly promoted fracture union at 21 days, as analyzed by radiography and μ-CT. The therapeutic effect appears to involve enhanced osteoblastic differentiation of MSCs and remodeling of callus tissues to laminar bone. This interpretation is supported by the enhanced expression of several key genes participating in the fracture repair process. In conclusion, AAV-DJ is a promising serotype for the AAV-based COX2 gene therapy of fracture repair in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Praemer A, Furner S, Rice D . In American Academy of Orthopaedic Surgeons. Musculoskeletal conditions in the United States. Park Ridge, IL, USA. Musculoskelet Injur 1992: 85–124.
Rundle CH, Strong DD, Chen ST, Linkhart TA, Sheng MH, Wergedal JE, et al. Retroviral-based gene therapy with cyclooxygenase-2 promotes the union of bony callus tissues and accelerates fracture healing in the rat. J Gene Med 2008; 10: 229–241.
Lau KH, Kothari V, Das A, Zhang XB, Baylink DJ . Cellular and molecular mechanisms of accelerated fracture healing by COX2 gene therapy: studies in a mouse model of multiple fractures. Bone 2013; 53: 369–381.
Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther 2005; 12: 212–218.
Evans CH . Gene therapy for bone healing. Expert Rev Mol Med 2010; 12: e18.
Tarassoli P, Khan WS, Hughes A, Heidari N . A review of techniques for gene therapy in bone healing. Curr Stem Cell Res Ther 2013; 8: 201–209.
Mietzsch M, Grasse S, Zurawski C, Weger S, Bennett A, Agbandje-McKenna M, et al. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1–12 vectors for gene therapy. Hum Gene Ther 2014; 25: 212–222.
Quinn K, Quirion MR, Lo CY, Misplon JA, Epstein SL, Chiorini JA . Intranasal administration of adeno-associated virus type 12 (AAV12) leads to transduction of the nasal epithelia and can initiate transgene-specific immune response. Mol Ther 2011; 19: 1990–1998.
Mori S, Wang L, Takeuchi T, Kanda T . Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology 2004; 330: 375–383.
Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA . Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol 2008; 82: 1399–1406.
Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol 2008; 82: 5887–5911.
Bartel M, Schaffer D, Buning H . Enhancing the clinical potential of AAV vectors by capsid engineering to evade pre-existing immunity. Front Microbiol 2011; 2: 204.
Yazici C, Takahata M, Reynolds DG, Xie C, Samulski RJ, Samulski J, et al. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther 2011; 19: 1416–1425.
Luo J, Sun MH, Kang Q, Peng Y, Jiang W, Luu HH, et al. Gene therapy for bone regeneration. Curr Gene Ther 2005; 5: 167–179.
Shah K, Majeed Z, Jonason J, O'Keefe RJ . The role of muscle in bone repair: the cells, signals, and tissue responses to injury. Curr Osteopor Rep 2013; 11: 130–135.
Li TF, Zuscik MJ, Ionescu AM, Zhang X, Rosier RN, Schwarz EM, et al. PGE2 inhibits chondrocyte differentiation through PKA and PKC signaling. Exp Cell Res 2004; 300: 159–169.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001; 25: 402–408.
Li N, Ghia JE, Wang H, McClemens J, Cote F, Suehiro Y, et al. Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol 2011; 178: 662–671.
Lau KH, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li Z, et al. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab 2013; 305: E271–E281.
Acknowledgements
This work is supported by the Telemedicine and Advanced Technology Research Center (TATRC) at the US Army Medical Research and Material Command (USAMRMC) under Grant No. W81XWH-12-1-0023. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lakhan, R., Baylink, D., Lau, KH. et al. Local administration of AAV-DJ pseudoserotype expressing COX2 provided early onset of transgene expression and promoted bone fracture healing in mice. Gene Ther 22, 721–728 (2015). https://doi.org/10.1038/gt.2015.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.40
This article is cited by
-
Ultramicroscopy as a novel tool to unravel the tropism of AAV gene therapy vectors in the brain
Scientific Reports (2016)