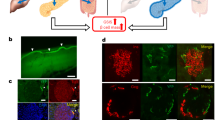
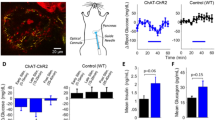
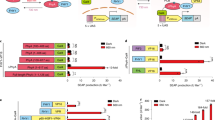
Abstract
The present study assessed the ability of optogenetics techniques to provide a better understanding of the control of insulin secretion, particularly regarding pancreatic β-cell function in homeostasis and pathological conditions such as diabetes mellitus (DM). We used optogenetics to investigate whether insulin secretion and blood glucose homeostasis could be controlled by regulating intracellular calcium ion concentrations ([Ca2+]i) in a mouse pancreatic β-cell line (MIN6) transfected with the optogenetic protein channelrhodopsin-2 (ChR2). The ChR2-transfected MIN6 (ChR2-MIN6) cells secreted insulin following irradiation with a laser (470 nm). The increase in [Ca2+]i was accompanied by elevated levels of messenger RNAs that encode calcium/calmodulin-dependent protein kinase II delta and adenylate cyclase 1. ChR2-MIN6 cells suspended in matrigel were inoculated into streptozotocin-induced diabetic mice that were then subjected to a glucose tolerance test. Laser irradiation of these mice caused a significant decrease in blood glucose, and the irradiated implanted cells expressed insulin. These findings demonstrate the power of optogenetics to precisely and efficiently controlled insulin secretion by pancreatic β-cells ‘on demand', in contrast to techniques using growth factors or chemical inducers. Optogenetic technology shows great promise for understanding the mechanisms of glucose homeostasis and for developing treatments for metabolic diseases such as DM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Guo JH, Chen H, Ruan YC, Zhang XL, Zhang XH, Fok KL et al. Glucose-induced electrical activities and insulin secretion in pancreatic islet beta-cells are modulated by CFTR. Nat Commun 2014; 5: 4420.
American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37 (Suppl 1): S81–S90.
National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Centres for Disease Control and Prevention, US Department of Health and Human Services, 2014.
Ashcroft FM, Rorsman P . Diabetes mellitus and the beta cell: the last ten years. Cell 2012; 148: 1160–1171.
Vetere A, Choudhary A, Burns SM, Wagner BK . Targeting the pancreatic beta-cell to treat diabetes. Nat Rev Drug Discov 2014; 13: 278–289.
Newby BN, Terada N, Mathews CE . In search of a surrogate: engineering human beta cell lines for therapy. Trends Endocrinol Metab 2014; 25: 378–380.
Marzorati S, Melzi R, Citro A, Cantarelli E, Mercalli A, Scavini M et al. Engraftment versus immunosuppression: cost-benefit analysis of immunosuppression after intrahepatic murine islet transplantation. Transplantation 2014; 97: 1019–1026.
Gerace D, Martiniello-Wilks R, O'Brien BA, Simpson AM . The use of beta-cell transcription factors in engineering artificial beta cells from non-pancreatic tissue. Gene Ther 2014; 22: 1–8.
Deisseroth K . Optogenetics. Nat Methods 2011; 8: 26–29.
Pastrana E . Optogenetics: controlling cell function with light. Nat Methods 2010; 8: 24–25.
Aravanis AM . An optical neural inteface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 2007; 4: S143–S156.
Arenkiel BR . In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 2007; 54: 205–218.
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K . Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005; 8: 1263–1268.
Han X, Boyden ES . Multiple-color optical activation, silencing and desynchronization of neural activity with single-spike temporal resolution. PloS One 2007; 2: e299.
Ishizuka T, Kakuda M, Araki R, Yawo H . Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res 2006; 54: 85–94.
Nagel G . Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 2005; 15: 2279–2284.
Petreanu L, Huber D, Sobczyk A, Svoboda K . Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 2007; 10: 663–668.
Schroll C . Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 2006; 16: 1741–1747.
Wang H . High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci USA 2007; 104: 8143–8148.
Zhang F . Multimodal fast optical interrogation of neural circuitry. Nature 2007; 446: 633–639.
Zhang F, Wang LP, Boyden ES, Deisseroth K . Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 2006; 3: 785–792.
Sineshchekov OA, Jung KH, Spudich JL . Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci US A 2002; 99: 8689–8694.
Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 2003; 100: 13940–13945.
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K . Millisecond-timescale genetically targeted optical control of neural activity. Nat Neurosci 2005; 8: 1263–1268.
Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci USA 2005; 102: 17816–17821.
Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 2007; 54: 205–218.
Lagali PS, Balya D, Awatramani GB, Munch TA, Kim DS, Busskamp V et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci 2008; 11: 667–675.
Tomita H, Sugano E, Isago H, Tamai M . Channelrhodopsins provide a breakthrough insight into strategies for curing blindness. J Genet 2009; 88: 409–415.
Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG et al. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci USA 2014; 111: 12913–12918.
Bryson JB, Machado CB, Crossley M, Stevenson D, Bros-Facer V, Burrone J et al. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science 2014; 344: 94–97.
Henquin JC . Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000; 49: 1751–1760.
Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S . The Cell Physiology of Biphasic Insulin Secretion. News Physiol Sci 2000; 15: 72–77.
Gembal M, Gilon P, Henquin JC . Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest 1992; 89: 1288–1295.
Borodinsky LN, Spitzer NC . Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci STKE 2006; 2006: pe22.
Seino S, Shibasaki T . PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 2005; 85: 1303–1342.
Mohlig M, Wolter S, Mayer P, Lang J, Osterhoff M, Horn PA et al. Insulinoma cells contain an isoform of Ca2+/calmodulin-dependent protein kinase II delta associated with insulin secretion vesicles. Endocrinology 1997; 138: 2577–2584.
Kitaguchi T, Oya M, Wada Y, Tsuboi T, Miyawaki A . Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. Biochem J 2013; 450: 365–373.
Rorsman P, Braun M, Zhang Q . Regulation of calcium in pancreatic alpha- and beta-cells in health and disease. Cell Calcium 2012; 51: 300–308.
Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes 2008; 57: 1618–1628.
Reinbothe TM, Safi F, Axelsson AS, Mollet IG, Rosengren AH . Optogenetic control of insulin secretion in intact pancreatic islets with beta-cell-specific expression of Channelrhodopsin-2. Islets 2014; 6: e28095.
Kushibiki T, Okawa S, Hirasawa T, Ishihara M . Optogenetics: novel tools for controlling mammalian cell functions with light. Int J Photoenergy 2014; 2014: 1–10.
Ye H, Daoud-El Baba M, Peng RW, Fussenegger M . A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 2011; 332: 1565–1568.
Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990; 127: 126–132.
Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M et al. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 1993; 36: 1139–1145.
Acknowledgements
This work was supported by The Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant-in-Aid Project for Scientific Research on Innovative Areas 'Crosstalk between transcriptional control and energy pathways, mediated by hub metabolites' (26116728) and Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Young Scientists (A) (25713009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Kushibiki, T., Okawa, S., Hirasawa, T. et al. Optogenetic control of insulin secretion by pancreatic β-cells in vitro and in vivo. Gene Ther 22, 553–559 (2015). https://doi.org/10.1038/gt.2015.23
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.23
This article is cited by
-
Optogenetic Approach in Trigeminal Neuralgia and Potential Concerns: Preclinical Insights
Molecular Neurobiology (2023)
-
Optogenetic regulation of insulin secretion in pancreatic β-cells
Scientific Reports (2017)
-
Shining Light on the Sprout of Life: Optogenetics Applications in Stem Cell Research and Therapy
The Journal of Membrane Biology (2016)