Abstract
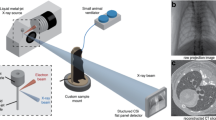
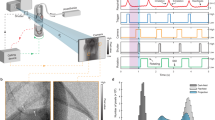
Although airway gene transfer research in mouse models relies on bolus fluid dosing into the nose or trachea, the dynamics and immediate fate of delivered gene transfer agents are poorly understood. In particular, this is because there are no in vivo methods able to accurately visualize the movement of fluid in small airways of intact animals. Using synchrotron phase-contrast X-ray imaging, we show that the fate of surrogate fluid doses delivered into live mouse airways can now be accurately and non-invasively monitored with high spatial and temporal resolution. This new imaging approach can help explain the non-homogenous distributions of gene expression observed in nasal airway gene transfer studies, suggests that substantial dose losses may occur at deliver into mouse trachea via immediate retrograde fluid motion and shows the influence of the speed of bolus delivery on the relative targeting of conducting and deeper lung airways. These findings provide insight into some of the factors that can influence gene expression in vivo, and this method provides a new approach to documenting and analyzing dose delivery in small-animal models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Liu C, Wong E, Miller D, Smith G, Anson D, Parsons D . Lentiviral airway gene transfer in lungs of mice and sheep: successes and challenges. J Gene Med 2010; 12: 647–658.
Stocker A, Kremer K, Koldej R, Miller D, Anson D, Parsons D . Single-dose lentiviral gene transfer for lifetime airway gene expression. J Gene Med 2009; 11: 861–867.
Cmielewski P, Anson DS, Parsons DW . Lysophosphatidylcholine as an adjuvant for lentiviral vector mediated gene transfer to airway epithelium: effect of acyl chain length. Respir Res 2010; 11: 84.
Limberis M, Anson DS, Fuller M, Parsons DW . Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Hum Gene Ther 2002; 13: 1961–1970.
Griesenbach U, Boyd AC . Pre-clinical and clinical endpoint assays for cystic fibrosis gene therapy. J Cyst Fibros 2005; 4: 89–100.
Griesenbach U, Smith SN, Farley R, Singh C, Alton EW . Validation of nasal potential difference measurements in gut-corrected CF knockout mice. Am J Resp Cell Mol 2008; 39: 490–496.
Soto-Montenegro ML, Conejero L, Vaquero JJ, Baeza ML, Zubeldia JM, Desco M . Assessment of airway distribution of transnasal solutions in mice by PET/CT imaging. Mol Imaging Biol 2009; 11: 263–268.
Cloetens P, Barrett R, Baruchel J, Guigay JP, Schlenker M . Phase objects in synchrotron radiation hard X-ray imaging. J Phys D: Appl Phys 1996; 29: 133–146.
Snigirev A, Snigireva I, Kohn V, Kuznetsov S, Schelokov I . On the possibilities of X-ray phase contrast microimaging by coherent high-energy synchrotron radiation. Rev Sci Instrum 1995; 66: 5486–5492.
Parsons DW, Morgan K, Donnelley M, Fouras A, Crosbie J, Williams I et al. High-resolution visualization of airspace structures in intact mice via synchrotron phase-contrast X-ray imaging (PCXI). J Anat 2008; 213: 217–227.
Siu KKW, Morgan KS, Paganin DM, Boucher R, Uesugi K, Yagi N et al. Phase contrast X-ray imaging for the non-invasive detection of airway surfaces and lumen characteristics in mouse models of airway disease. Eur J Radiol 2008; 68: S22–S26.
Donnelley M, Morgan K, Fouras A, Skinner W, Uesugi K, Yagi N et al. Real-time non-invasive detection of inhalable particulates delivered into live mouse airways. J Synchrotron Radiat 2009; 16: 553–561.
Donnelley M, Morgan K, Skinner W, Suzuki Y, Takeuchi A, Uesugi K et al. A new technique to examine individual particle and fibre deposition and transit behaviour on live mouse trachea. J Synchrotron Radiat 2010; 17: 719–729.
Sinn PL, Arias AC, Brogden KA, McCray PB . Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J Virol 2008; 82: 10684–10692.
Mitomo K, Griesenbach U, Inoue M, Somerton L, Meng CX, Akiba E et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with sendai virus envelopes. Mol Ther 2010; 18: 1173–1182.
Hart D, Hillier MC, Wall BF . National reference doses for common radiographic, fluoroscopic and dental X-ray examinations in the UK. Br J Radiol 2009; 82: 1–12.
Kitchen MJ, Paganin D, Lewis RA, Yagi N, Uesugi K, Mudie ST . On the origin of speckle in X-ray phase contrast images of lung tissue. Phys Med Biol 2004; 49: 4335–4348.
Grubb BR, Boucher RC . Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev 1999; 79 (1 Suppl): S193–S214.
Chandler SG, Illum L, Thomas NW . Nasal absorption in the rat. 1. A method to demonstrate the histological effects of nasal formulations. Int J Pharmaceut 1991; 70: 19–27.
Sinn PL, Burnight ER, Hickey MA, Blissard GW, McCray Jr PB . Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J Virol 2005; 79: 12818–12827.
Mery S, Gross EA, Joyner DR, Godo M, Morgan KT . Nasal diagrams—a tool for recording the distribution of nasal lesions in rats and mice. Toxicol Pathol 1994; 22: 353–372.
Cmielewski P, Anson DS, Parsons DW . Re-emergence of luciferase expression in lung following a single nasal instillation of a lentiviral vector in normal and cystic fibrosis mice. J Cyst Fibros 2010; 9 (S1): S17.
Grubb BR, Rogers TD, Boucher RC, Ostrowski LE . Ion transport across CF and normal murine olfactory and ciliated epithelium. Am J Physiol Cell Physiol 2009; 296: C1301–C1309.
Boone JM, Velazquez O, Cherry SR . Small-animal X-ray dose from micro-CT. Mol Imaging 2004; 3: 149–158.
Figueroa SD, Winkelmann CT, Miller WH, Volkert WA, Hoffman TJ . TLD assessment of mouse dosimetry during microCT imaging. Med Phys 2008; 35: 3866–3874.
Griesenbach U, Munkonge FM, Sumner-Jones S, Holder E, Smith SN, Boyd AC et al. Assessment of CFTR function after gene transfer in vitro and in vivo. Methods Mol Biol 2008; 433: 229–242.
Gallotti A, Uggeri F, Favilla A, Cabrini M, de Haen C . The chemistry of iomeprol and physico-chemical properties of its aqueous solutions and pharmaceutical formulations. Eur J Radiol 1994; 18 (Suppl 1): S1–S12.
Iomeron Data Sheet. From: Eisai Co., Ltd, Japan, 2009, http://www.eisai.jp/medical/products/di/EPI/IOM_V_EPI.pdf.
Griesenbach U, Meng C, Farley R, Wasowicz MY, Munkonge FM, Chan M et al. The use of carboxymethylcellulose gel to increase non-viral gene transfer in mouse airways. Biomaterials 2010; 31: 2665–2672.
Sinn PL, Shah AJ, Donovan MD, McCray Jr PB . Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am J Resp Cell Mol 2005; 32: 404–410.
Goto S, Takeshita K, Suzuki Y, Ohashi H, Asano Y, Kimura H et al. Construction and commissioning of a 215-m-long beamline at SPring-8. Nucl Instrum Meth A 2001; 467: 682–685.
Yabashi M, Yamazaki H, Tamasaku K, Goto S, Takeshita K, Mochizuki T et al. SPring-8 standard X-ray monochromators. Proc SPIE 1999; 3773: 2–13.
Donnelley M, Parsons D, Morgan K, Siu K . Animals in synchrotrons: overcoming challenges for high-resolution, live, small-animal imaging. AIP Proc 2010; 1266: 30–34.
Acknowledgements
This study was supported by the NH&MRC Australia, with additional support from philanthropic donors via the CURE4CF Foundation (http://www.cure4cf.org). The synchrotron radiation experiments were performed on the BL20B2 beamline at SPring-8, with the approval of the Japan Synchrotron Radiation Institute (JASRI) under proposal number 2010A1523. Professor Naoto Yagi and Dr Kentaro Uesugi provided expert synchrotron imaging and controls advice and assistance at the SPring-8 synchrotron. We thank P Cmielewski, M Limberis and D Miller for their editorial input. MD, KS, AJ and DP were supported by the International Synchrotron Access Program (ISAP) managed by the Australian Synchrotron. The ISAP is an initiative of the Australian Government being conducted as part of the National Collaborative Research Infrastructure Strategy.
Author contributions
DP conceived the research; MD and DP devised the experiments; MD and DP performed the animal experiments in conjunction with KS and AJ who performed the synchrotron imaging; MD and DP conceived the image analyses; MD performed the image analyses and constructed the figures and Supplementary Material; MD and DP wrote the initial manuscript and all authors edited and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Donnelley, M., Siu, K., Jamison, R. et al. Synchrotron phase-contrast X-ray imaging reveals fluid dosing dynamics for gene transfer into mouse airways. Gene Ther 19, 8–14 (2012). https://doi.org/10.1038/gt.2011.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.80
Keywords
This article is cited by
-
Improved in-vivo airway gene transfer via magnetic-guidance, with protocol development informed by synchrotron imaging
Scientific Reports (2022)
-
In vivo Dynamic Phase-Contrast X-ray Imaging using a Compact Light Source
Scientific Reports (2018)
-
Transduction of ferret airway epithelia using a pre-treatment and lentiviral gene vector
BMC Pulmonary Medicine (2014)
-
Non-invasive airway health assessment: Synchrotron imaging reveals effects of rehydrating treatments on mucociliary transit in-vivo
Scientific Reports (2014)