Abstract
Viral therapy of cancer includes strategies such as viral transduction of tumour cells with ‘suicide genes’, using viral infection to trigger immune-mediated tumour cell death and using oncolytic viruses for their direct anti-tumour action. However, problems still remain in terms of adequate viral delivery to tumours. A role is also emerging for single-organ isolation and perfusion. Having begun with the advent of isolated limb perfusion for extremity malignancy, experimental systems have been developed for the perfusion of other organs, particularly the liver, kidneys and lungs. These are beginning to be adopted into clinical treatment pathways. The combination of these two modalities is potentially significant. Locoregional perfusion increases the exposure of tumour cells to viral agents. In addition, the avoidance of systemic elimination through the immune and reticulo-endothelial systems should provide a mechanism for increased transduction/infection of target cells. The translation of laboratory research to clinical practice would occur within the context of perfusion programmes, which are already established in the clinic. Many of these programmes include the use of vasoactive cytokines such as tumour necrosis factor-α, which may have an effect on viral uptake. Evidence of activation of specific anti-tumour immunological responses by intratumoural and other existing methods of viral administration raises the intriguing possibility of a locoregional therapy, with the ability to affect distant sites of disease. In this review, we examined the state of the literature in this area and summarized current findings before indicating likely areas of continuing interest.
Similar content being viewed by others
Introduction
Genetic manipulation of tumours is an attractive proposition to those involved in cancer therapeutics. Traditional therapies such as chemotherapy and radiotherapy rely on an ability to affect cellular replication and survival, usually by causing DNA damage or impairing the orderly progression of normal cell-cycle events. However, the poor response rates of some tumour types to conventional therapy and a desire for more efficient, pin-point tumour destruction has led to a proliferation of ‘targeted’ agents against specific proteins known to be involved in the pathogenesis of cancer. These drugs may act on pathways implicated in cancer cell growth, survival and immortalization, blood supply or spread. These targeted agents aim either to exert a direct cytotoxic effect or to act as ‘sensitization’ agents for other therapies. As with all therapies, the aim is to develop an agent with maximal efficacy against tumour cells but with minimization of the deleterious effects on normal human tissue and consequent toxicity.
At the root of all tumours is a set of mutations in the genome of the malignant cell that causes it to behave differently to the surrounding genotypically normal cells. Therefore, approaches that can selectively alter the proteins expressed by cancer cells1 or exploit metabolic differences between them and normal cells to allow virus propagation are attractive. The first of these uses DNA as a quasi-therapeutic molecule that does not exert a direct anti-tumour effect but, rather, is transcribed and translated into a protein product that can mediate tumour cell death. Classical examples of this type of gene therapy include restoration of tumour suppressor gene function, abrogation of oncogene activation, direct provision of endogenous or exogenous genes that have a direct cytoreductive effect and gene delivery to induce anti-tumour immune responses.2 However, in the last decade, it has become clear that certain abnormalities in signal transduction pathways in cancer cells make them fertile soil for the infection and replication of a range of viruses. A number of these viruses (such as adenovirus, alphaviruses, herpes simplex virus, measles virus, reovirus, vaccinia virus, vesicular stomatitis virus) show tumour-selective replication and cytotoxicity—properties that have been recognized by the collective term ‘oncolytic viruses’.3 In this situation, the genetic material of an oncolytic virus can act therapeutically simply by directing the normal lytic viral life cycle in tumour (but not normal) cells. In addition, such viruses can be genetically manipulated to arm them with additional classical gene therapy capabilities (as detailed above).
With replication-competent agents, the normal viral life cycle means that an absolute requirement of viral function is to infect cells and subjugate them to the task of replicating viral genomes and proteins. Genetic manipulation techniques allow researchers to alter the genome of viral particles to include genes encoding for specific proteins or other moieties. In the case of ‘suicide’ gene therapy, the product of the novel DNA is cytotoxic, or can be combined with an inert, co-administered substance to produce an anti-tumour effect in both transduced and untransduced (bystander effect) cells.4, 5 However, viruses need not be limited to the role of mere molecular delivery agents. Some viruses possess, as part of their life cycle, an ability to lyse host cells and, thereby, have the potential to be used as direct cytotoxic agents in their own right.6 Finally, viral infection of host cells can produce a potent immunostimulatory effect as the host begins to counter infection and, if this can be harnessed to alert the immune system to the presence of a tumour by exposure to tumour-associated antigens that are present within a mutated cell, the induction of host anti-tumour immunity becomes feasible. Such an approach has shown limited success in the context of tumour vaccines.7
In addition to direct effects, viruses have shown an ability to be complementary to existing therapies. Oncolytic Reovirus, among others, has shown promise as a sensitizer to radiotherapy,8, 9 and similar effects have been observed with cytotoxic chemotherapy.10, 11 Administration of an oncolytic virus has also been shown to prime immune responses in distant lymphatic organs.12
The ideal viral vector should have a number of characteristics. First, it should be easily administered in a similar manner to existing agents—intravenous (or intravascular) delivery is ideal. It should exhibit a targeted effect that is limited to tumour cells and carry minimal threat to normal tissue. Next, it must be possible to administer viral titres that can treat both primary and metastatic diseases (or there must be sufficient in vivo viral replication/amplification to achieve this goal). The virus or its vehicle must evade non-specific immune-mediated degradation. Finally, it should be able to prime host anti-tumour immune responses. Although some of these facets are functions of the virus chosen, others can be modulated by the method of administration.13
As yet, systemic administration of oncolytic viruses has not fully lived up to the promise suggested by preclinical studies, because of the difficulties inherent in the systemic administration of the virions. These include non-specific absorption and destruction by the reticulo-endothelial system and specific immune response (antibody neutralization) brought about by the previous immunity of subjects to such agents.
The role of the immune system in the context of oncolytic therapy is complex. On the one hand, immune destruction of virus after systemic administration may significantly reduce doses reaching tumours;14 on the other, a functional immune system is vital for maximal anti-tumour effect, with CD8+ and Natural Killer cells paramount.15 Although de Wilt et al.16 noted an increase in systemic histamine levels and both systemic and intratumoural leukocytosis after isolated limb perfusion (ILP) in rats, there exist little data to quantify and define the immune response after locoregional viral administration. However, given the increasing recognition of the central role of immune modulation, it is well described for both intravenous and intratumoural injections. After intratumoural injection, a rapid acute inflammatory response results in high levels of inflammatory cytokines both locally within the tumour and also at regional lymph nodes. This is followed by a significant increase in T-cell activation, with specificity for the dominant viral antigens most easily detected. These effects are likely to be tumour type specific, and do not always depend on the ongoing intratumoural viral replication.15 Similar increases in intravenous cytokine levels and T-cell activation are encountered after systemic administration, but alongside these, comes the development of neutralizing antibodies, which are seen at day 5 and peak between 7 and 14 days after viral administration.17
Clearly, after isolated organ perfusion, a degree of immune stimulation is not only inevitable but also desirable. Further studies evaluating the timing, magnitude and modulation of this response by locoregional administration techniques will be essential.
In the Aethiopis, attributed to Arctinus of Miletus, Achilles is finally killed by Paris's arrow, directed at his one spot of weakness—his heel, which was not immersed in the River Styx. Systemic therapy represents a sheaf of arrows fired in the hope of hitting the appropriate target within tumour cells; however, isolation perfusion may offer a mechanism by which our therapeutic ‘arrows’ can be better directed to the weak point(s) of tumour biology that they seek to exploit.
The role of surgery in viral tumour therapy: locoregional perfusion
Many patients with cancer undergo surgery. In most cases, this is with curative intent but it has long been recognized that a group of patients will already have micro-metastatic disease at presentation that is not detectable by current clinical and imaging assessment.18, 19 The presence of such metastatic disease is universally associated with a poorer prognosis. There is also a role for surgery in a palliative setting, when disease resection can be beneficial in terms of quality of life or local disease control.20 The most obvious example of this latter group is provided by patients with severe in-transit disease (AJCC (the American Joint Committee on Cancer) stage IIIb or above21) from malignant melanoma. No intervention, from adjuvant therapies to sentinel lymph node biopsy and elective nodal dissection,22 has been shown significantly to increase survival in this group. However, the surgical procedures that the patients undergo, including ILP, for which this is a major indication, may open up new avenues for administration of viral agents.
Isolated locoregional perfusion has been a useful concept in cancer therapeutics for many years, since Creech et al.23 published their early experiences in 1958. Subsequently, others have described the necessary features for successful perfusions, and the scope of treatment has widened from the initial limb perfusion to include single-organ perfusion.24 Briefly, perfusion could be considered possible in any end organ in which the arterial inflow and venous outflow are provided by vascular systems that can be easily controlled. This is particularly true in the post-cytokine era; tumour necrosis factor-α (TNFα) was first introduced into the perfusion system by LeJeune and colleagues25 in the 1990s, but given its extremely severe systemic side effects, requires effective control of both sides of the perfusion and hence reduction in systemic leakage. The agent of interest is administered by addition to the perfusate within the reservoir (Figure 1). It should be noted that the presence of multiple inflows, as occurs with the portal system in hepatic perfusion, is not per se a barrier to perfusion but increases the technical difficulty in both animal and human subjects.
As perfusion is not limited to organs but rather to an area with a defined inflow and outflow, free tissue flaps, which are commonly used by plastic and other surgeons in the reconstructive process after radical surgery, are also potential targets for intravascular therapy.26 A further theoretical attraction of this approach is that transduction of a flap with virus may provide a mechanism for local action on microscopic residual disease beyond the flap at the tumour bed and, hence, reduce local recurrence. Overall, animal studies have shown that the effective concentrations of a chemotherapeutic agent achievable within target organs by perfusion-type systems are typically 15–25 times those tolerated in systemic administration,27, 28, 29, 30, 31 in which dosages are limited by systemic toxicity. The maximum level of chemotherapeutic attainable within the tumour is heavily dependent on the physical and temporal characteristics of perfusion. Maximal doses are achieved after bolus injection into the perfusate reservoir during ILP at 38–41 °C. This provides a 60% advantage over normothermic perfusion and ‘split-dosing’, wherein melphalan is administered throughout the perfusion as a series of fractional doses.32 However, hyperthermia also increases the locoregional toxicity of cytotoxic agents, such that moderate hyperthermia of 38–39 °C is the aim of clinical perfusion. In addition, because the isolation and subsequent washout of vessels effectively eliminates systemic exposure, additional vasoactive factors such as cytokines can be added to increase vascular permeability at the site of the tumour.30, 31 Such cytokines are profoundly toxic when administered systemically, but are well tolerated during isolated perfusions assuming that systemic leak is prevented.
Despite the theoretical advantages of isolated organ perfusion described (Table 1), several randomized trials of locoregional administration versus systemic therapy have failed to show benefits in terms of increased long-term survival. This is true both in the prophylactic setting33 and in the treatment of hepatic tumours by repeat hepatic artery infusion (HAI).34 Thus, although impressive local response rates are observed,35, 36 there is currently a difficulty in converting the theoretical advantages of locoregional perfusion into long-term clinical improvements. However, currently, ILP has been adopted as a treatment strategy across much of Europe for locally advanced sarcoma and melanoma. Regional perfusion in other areas is lagging behind ILP, but recently has been used clinically in the pelvis,37 liver38 and, in related forms, the brain.39 In addition, isolated limb infusion is growing in clinical application in Australasia40 and other centres.41
In viral therapy, the potential for protection of virus from the systemic immune system for the duration of the perfusion, combined with the ability to artificially modify the tumour vasculature and increase intratumoural penetration of viral agents, offers a hope for increased infection of target tumours. TNFα is not the only cytokine that has shown synergy in vivo with chemotherapeutics, with a similar effect seen with interleukin-2.42 In addition, interleukin-2 has been used in systemic administration trials to provide immunomodulation and further enhance viral efficacy.43 It is possible that other substances, such as histamine, which has synergistic effects in perfusion model systems with chemotherapeutic,44 may also enhance viral action. Isolation perfusion offers a mechanism to increase the doses of these vasoactive substances without deleterious systemic effects, and the complex interactions between cytokines, viruses and chemotherapeutics may be best elucidated in such models.
Other locoregional administration techniques
In addition to isolated organ perfusion, the advances in interventional radiology have opened up new avenues of locoregional perfusion therapies. Intra-arterial infusion, whereby a therapeutic substance is injected directly into the artery supplying the organ (and tumour), shares many of the characteristics of perfusion but has a few obvious drawbacks.24, 45 First, although high ‘first-pass’ concentrations can be achieved at the tumour site, the agent is rapidly diluted within the systemic circulation and there is minimal protection from metabolic (for chemotherapeutic agents) or immune destruction in subsequent circulation. The use of balloon exclusion catheters has also been considered. Although the principles are similar to ILP, in practice, these catheters can only exclude single vessel flow. Therefore, although some initial promise was seen in animal studies in pig models, human procedures were rapidly abandoned after significant leakage was observed through collateral circulation in an attempted pelvic perfusion.24, 46, 47 The difficulty was likely to have been control of the venous return; as application of a tourniquet to the supra- and infra-hepatic vena cava necessitates a laparotomy, the minimally invasive benefits of a balloon catheter approach would have been negated. It may be possible to adapt the approach for ILP, in which the collateral venous return is controlled using a tourniquet.
A further approach to locoregional administration is the use of intraperitoneal delivery for chemotherapy in patients with peritoneal surface disease in various primary tumour types. Intraperitoneal delivery of chemotherapeutics has been used for some time,48 but peritoneal perfusion is now being adopted into clinical practice with increasing regularity. At present, various chemotherapeutic agents (determined by tumour type) are administered, either concomitantly with traditional cytoreductive surgery or in the adjuvant setting after insertion of perfusion cannulas at laparotomy. A similar perfusion system to that followed in ILP is used, with flow rates of 1 l min−1.49 Three randomized controlled trials showed improved median survival in patients with ovarian carcinoma after intraperitoneal administration50, 51, 52 and subsequent experience has shown similar improvements for peritoneal surface disease of appendiceal, colorectal, gastric, small bowel and sarcomatoid origin, as well as in abdominal mesothelioma.53 The main concern preventing the uptake of this procedure has been the potential for major morbidity and mortality, as well as availability of high-volume centres with appropriate multi-disciplinary experience. A review by Chua et al.54 points out the similarity between the morbidity and mortality of HIPEC (Hyperthermic IntraPEritoneal Chemotherapy) with that of a Whipple procedure, which was similarly pilloried for excessive complications at its inception. Mortality rates between 0.9 and 5.8%, with a surgical morbidity of 12–52%, can be achieved. The average intensive care unit stay is 1–5 days, with a total hospital stay of 7–48 days.49, 55 These figures are not wildly removed from those for major upper gastrointestinal and hepatobiliary oncological surgery, and the survival advantage gained is equivalent or better. For ovarian carcinoma, median progression-free survival improves from ∼22 to 28 months, with a gain in median overall survival from 49.7 to 65.6 months.50, 51, 52 As would be expected, response and hence survival rates vary with primary pathology and resection status, but similar gains are observed in other primary tumour types54 (Table 2).
Gene therapy in isolated perfusion models
The majority of data about the feasibility of an isolated perfusion approach to tumour virotherapy comes from animal models. This is predominantly because the surgical techniques for more complex human organ perfusion have only relatively recently made the transition from long-standing animal model to human clinical settings and have not yet been optimized for human use.64
Technical features of viral isolated perfusion
Many fluids have been used in perfusions, ranging from simple crystalloid solutions (saline, Ringer's Lactate) to complex colloids, such as the UW (University of Wisconsin) solution. The UW solution was developed for cold perfusion of transplant organs and, therefore, many papers evaluating viral delivery with this fluid use transplantation models. However, it would seem likely that some of these results will transfer to the cancer therapy setting. Henry et al.65 evaluated the additional benefit of hydroxyethyl starch as a colloid to viral transduction in the setting of cold-perfusion preservation of liver transplants, and discovered that the hydroxyethyl starch solutions increased viral transduction threefold. This effect could be abolished by the electrochemical neutralization of perfusion solution, making it likely that the slight negative charge of the UW solution at physiological pH was responsible for the increased transduction. Another advantage of colloids as perfusate fluid is the reduction in tissue oedema, as a result of the increased intravascular oncotic pressure exerted by large molecules. The use of fluid as the perfusate base represents a further modification of viral delivery. Baker et al.66 summarized the interaction of adenovirus with blood components, and work in our own laboratory confirms that vaccinia associates with the cellular component of whole blood, rather than being free in the serum (Pencavel T et al., unpublished observations). Thus, the relatively hypocellular nature of the perfusion field may confer a transduction/infection advantage for viral vectors.
‘Standard’ ILP in both human and animal subjects uses hyperthermia, which has been shown to increase the cytotoxicity of perfused agents at temperatures between 38 and 41 °C. Although few studies have evaluated the effect of temperature on efficiency of viral infection, one study did note that hypothermia at 4 °C was associated with a decrease in efficacy.67 This was believed to be due to the decreased kinetics of physical interactions between adenovirus and integrins on the cell surface seen at temperatures below 10 °C.68 Hyperthermia at 41.5 °C in a mouse tumour model also increased specific tumour uptake of Vaccinia virus within tumours by 100-fold.69 Therefore, hyperthermic perfusion of an organ or limb is likely to increase tumour viral uptake, while at the near-physiological temperatures used clinically there would be minimal effect on viability.
Perfusion pressure may also be important. Large particles, such as Sendai virions, can be ‘forced’ through narrow vascular openings by increasing the perfusion pressure. However, in the experimental setting, pressure must be closely controlled as it may alter outcome measures independently of viral effects by inducing pressure-related tissue necrosis70 and increase treatment toxicity.71
The final factor in perfusate composition, the viruses themselves, can be presented in a number of different ways to increase efficacy. Both oncolytic and vector strategies have been reported, with neither approach showing greater in vivo promise than the other. Doses of any vector below 1 × 105 have not been shown to be effective, whereas the highest tolerated dose, 1 × 1012 p.f.u. (plaque-forming units) of a replication-deficient adenovirus, represents a similar dose to that tolerated systemically.72, 73
Specific organ perfusion
Gene therapy is a relatively new development and many studies, both animal and human, have been directed at direct local or systemic administration. Thus, although 65% of gene therapy trials are currently directed at cancer therapeutics,74 a very limited number of these have used locoregional administration techniques, probably because they represent complex methods with only a small number of institutions worldwide performing perfusion procedures in humans. However, it is possible to extrapolate data from related fields to malignant disease, which in combination with published anti-cancer studies provide an indication of the potential utility of perfusion in this setting.
Isolated hepatic perfusion
Hepatic perfusion is an attractive prospect as it provides a mechanism to treat unresectable metastatic disease, for example, from colorectal cancer, as well as primary hepatocellular carcinoma.64, 75 Isolated hepatic perfusion (IHP) is more technically demanding than ILP, as major venous structures are harder to control surgically (Figure 2), given their relatively fragile thin vessel walls and the ensuing potential for problematic haemorrhage. In IHP, both the portal and vena caval systems must be controlled; however, this can be accomplished both ex vivo and in situ.
Arterial anatomy in the human (a) and rodent (b) limb. Venous return is more complex, but the vessels cannulated run with the arterial supply. Sites of cannulation are marked with arrows. Tumours in human ILP may be at any site in the limb distal to the inguinal ligament (dotted line, both pictures), with the most common site of implantation for animal models indicated in panel b. (c) A schematic of rodent hepatic perfusion is shown. The anatomy is identical to that for human IHP.
In general, two modelling systems have been used for IHP. The first, classical method involves cannulation of the hepatic artery and portal vein, with venous return through the vena cava. Viral administration occurs either through the arterial or portal inflow. A second method used is intrasplenic injection, using the spleen as a portal ‘reservoir’ to provide virus. This method is more practical in mouse models than IHP because of the size of the vessels involved, and, hence, allows for easier use of nude animals and a greater range of tumour types. de Roos et al.76 compared the two methods of administration using a hepatotropic adenoviral vector for transfer of a luciferase reporter gene under the control of a CMV (cytomegalovirus) promoter. The dose administered was 2 × 109 p.f.u. in both cases. Although both methods achieved some infection of hepatocytes, the degree of marker protein production was significantly improved by hepatic perfusion compared with splenic injection and was more reliable. Extrahepatic spread, quantified by extrahepatic luciferase expression, was found in both groups but at a lower level in the IHP group. In particular, activity within the testes of the rats was 10-fold lower after IHP than splenic injection, implying a reduced risk of germline transmission or mutation.
Similarly, Nomura et al.77 compared systemic administration through the tail vein with intraportal delivery through the spleen of an oncolytic herpes simplex virus agent against colorectal metastases. They described a significant increase in survival with both methods, with a concomitant reduction in metastatic burden as measured by liver weight. Although not a significant difference, liver weight was, on average, lower in the portal injection group. Assessment of viral load within metastases by quantitative PCR showed a higher viral load in the portal administration group. This provides some evidence for the ability of locoregional administration to avoid non-target site attenuation and provide a higher viral titre to the tumours. Interestingly, both they, and others, found no evidence of extrahepatic viral infection. Indeed, a study with a replication-competent murine leukaemia retrovirus armed with yeast cytosine deaminase found virus by PCR only within the metastases.78, 79 These studies indicate the ability of a locoregional technique to produce specific infection within tumour cells, with minimal non-target site infection, at higher levels than systemic administration. Further proof of this hierarchy of administration methods was provided in an analysis of the administration of armed adenoviral vectors encoding LacZ or anti-p21ras antibody by intratumoural injection, IHP, HAI or intravenous infusion.80 The model used was hepatic colorectal cancer metastases. The greatest level of infection was provided by intratumoural injection at 5%, with IHP providing 2–3% infection of target cells. No infection was seen after single or multiple HAI or intravenous administration, although there was some evidence of infection of tumour vasculature after multiple HAI. However, the active virus (anti-p21ras antibody encoding) only produced an objective tumour response after five administrations by HAI, likely to be due to its effects on the tumour vasculature rather than a direct anti-tumour response.
Isolated limb perfusion
Clinically, ILP is used predominantly for the treatment of melanoma and sarcoma.36 In the laboratory environment, it provides an easier method of assessing locoregional delivery than IHP as the vessels cannulated (the femoral artery and the vein) are accessible without a laparotomy, are generally of sufficient size as to be cannulated relatively easily with the aid of a standard operating microscope and, because of the field of perfusion, tumours treated tend to be intramuscular or subcutaneous and hence amenable to non-invasive, in vivo imaging techniques for the assessment of tumour response. Isolation of the collateral supply of the limb is accomplished using a tourniquet, removing the need for surgical control of additional vessels.
Isolated limb infusion is a closely related technique during which smaller cannulas are placed under radiological guidance and a non-oxygenated low-flow circuit is used.41, 81, 82 However, it has the disadvantage that in the clinical setting, it can only be used to administer cytotoxic agents and vasoactive agents such as TNFα cannot be used.83 Therefore, its applicability in viral models is more limited than ILP.
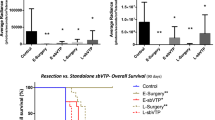
Viral infection studies require a reliable standard of infection with which to compare infection by alternative routes. In the context of ILP, with its cutaneous or intramuscular tumour location, this function is normally fulfilled by intratumoural injection of virus. However, the attraction of organ perfusion is the possibility of a more homogeneous spread of exposure to virus as a result of the use of the tumour's own vasculature as a distributory network.84 This has been shown to be the case in the setting of ILP using an armed adenoviral vector encoding a cytokine, with routes of administration including ILP, intratumoural injection and intravenous infusion. The dose of vector used ranged from 1 × 105 to 1 × 109 p.f.u. No response was seen at the lowest doses. However, at higher doses, no effect was seen on tumour growth when virus was administered intratumourally or intravenously, compared with a response rate of 4 of 9 animals (44%) by ILP to a rat osteosarcoma and 89% to the BN-175 soft tissue sarcoma line. Interestingly, the effect of melphalan with virus perfusion was not significantly different from that of melphalan with TNFα.16 On the basis of published evidence of synergy between melphalan and TNFα,85, 86 the latter strategy has become routine in clinical practice, and although no direct evidence has yet been found of synergism between virus and TNFα, any increase in viral penetrance by co-administration of cytokine may increase the overall response rate to perfusion. Other groups have since confirmed that the expression of virus-encoded marker protein within tumours is indeed more homogeneous than the ‘needle-track’ pattern identified after intratumoural injection.87 With regard to the biodistribution of viral products after ILP, most studies demonstrate little, if any, infection outside the perfusion field, with the exception of detection of hepatotropic adenoviral products in the liver in one study.88 In their efforts to define an optimal administration method for viral therapy using an adenoviral vector encoding luciferase or LacZ at a dose of 1 × 109 p.f.u., de Roos et al.89 defined a perfusion period of 15 min. They described a greater homogeneity of tumour infection, associated with significantly less non-target ‘leakage’ than was seen with intratumoural, intravenous or femoral artery infusion systems. This study also showed strong tumour selectivity to the viral agent, with much less muscular infection when compared with tumoural titres.
Perhaps the study with the most immediate applicability to human systems was performed at the National Institute of Health, where rhesus monkeys were perfused with varying doses and types of vaccinia virus.90 As none of the monkeys had tumours implanted, vaccinia, which underwent preferential replication only in tumour cells (that is, tumour-selective strains), could not be used as a model in studies attempting to evaluate the systemic toxicity of locally administered, actively replicating virus. Hence modified, replication-competent WR (Western Reserve) vaccinia with a LacZ gene insertion (vF13) was used as a surrogate. This strain is capable of rapid replication in normal tissues. Toxicity after systemic administration was established both for this strain and a double-deleted tumour-selective virus (vvDD). Unsurprisingly, a relatively low dose (1 × 108 p.f.u. intravenously) of the vF13 strain proved fatal, with systemic features of pox infection. However, the tumour-selective virus did not cause systemic manifestations at the same dose. After ILP with vF13, the cutaneous pox lesions were restricted only to skin below the tourniquet and did not spread proximally in subsequent days. Toxicity after ILP with vvDD occurred in the form of a rash limited to the perfusion field in an animal perfused at 1 × 1010 p.f.u., equivalent to a systemic dose, but only low levels of virus were recovered from the rash. Finally, the effect of previous vaccination was evaluated and proven to be reversible if perfusion was carried out after ‘wash out’ of native blood and proceeded using donated blood from a non-vaccinated animal. This exhaustive series of experiments holds valuable lessons for those planning viral therapy studies in human subjects; first, perfusion seems to offer a safer method of administration, even of wild-type viruses, than intravenous systemic administration; second, the viruses are restrained within the perfusion field and do not show any ability to move beyond that field in non-human primates; third, the doses administered through ILP, although directly comparable with systemic doses, are better tolerated; and finally, the perfusion system can be modified to optimize tumour penetrance.
Cardiovascular and pulmonary perfusion
Malignant disease of the cardiovascular system is among the rarest cancers known, and therefore modelling systems evaluating anti-tumour efficacy are not generally used. However, it is possible that viral therapy could be targeted against tumour vasculature rather than malignant cells themselves, and therefore lessons learnt from perfusion of transplanted pig hearts and isolated sections of the carotid artery may be instructive. In particular, O'Donnell and Lewandowski72 evaluated the transduction efficacy of AdV.CMV.LacZ in normal pig cardiac muscle by three mechanisms: aortic cross-clamp with ‘indwelling’ perfusate fluid (that is, the perfusate is not circulated but administered and then washed out), perfusion of the coronary vessels and perfusion of the coronary vessels after a wash-out period of host blood, in which the coronary vasculature was first purged of native blood cells by a brief period of saline perfusion with the effluent being discarded rather than re-circulated. The last approach is supported by analysis of the effects of blood on adenoviral vectors.66 They found viral product expression in 5, 23 and 58% of perfused cardiac smooth muscle respectively, but it must be noted that the second method produced the greatest homogeneity of transduction. In addition, only the first method led to the extracardiac detection of virus. Transduction rates of 23% have also been reported elsewhere,67 this time in the context of hypothermic perfusion, and the authors admit that the hypothermic perfusion sacrificed some transduction efficiency to improve the model's relevance to clinical transplantation. Finally, transduction rates of an alkaline phosphatase ALP-producing adenoviral vector of up to 35% were seen in the smooth muscle of carotid vessels of rabbits after an indwelling method was used.91 The authors noted no correlation between the length of viral exposure and transduction, but again a higher pressure was linked to greater viral gene expression. These studies demonstrate the ability of viruses to target the vasculature as well as tumour cells and, hence, may have wider implications for tumour therapy. All these studies were evaluating transduction/infection of the smooth muscle (either cardiac or vascular). However, there is evidence to suggest that viral infection in the context of tumour therapy may mediate some of its effects by infection and consequent destruction of the vascular endothelium.92 This is an effect that is likely to be increased when combined with cytokines which themselves possess an anti-tumour-associated vasculature effect.
In a study of herpes simplex virus acting against sarcoma metastases within the lungs, liver and bladder, Brooks et al.93 used both indwelling and perfusion methods. Despite initially starting with indwelling solutions of virus within the lung, this approach was abandoned when it became clear that both tumour and normal cells were adversely affected by the hypoxic period. Therefore, continuous perfusions were performed, with successful reduction in tumour burden as assessed by nodule count in the perfused compared with the non-perfused lung. They found no difference between ‘armed’ (TNF-producing) and ‘marker’ (β-galactosidase-producing) virus; therefore, the majority of the effect was attributed to the direct oncolytic action of the herpes simplex virus.
Conclusions
Pre-clinical studies have identified isolated organ perfusion as a successful modality for the infection/transduction of cells within the perfusion field (Table 3). By controlling the physiological parameters of the perfusion circuit, viral extravasation and therefore cellular infection can be manipulated to provide the optimum conditions for anti-cancer therapy. These mechanisms may therefore lead to more widespread applications of gene therapy for cancer that have been promised by encouraging early in vivo trials and provide another element in the armamentarium of surgeons and oncologists in the clinic.
References
Vucic D, Fairbrother WJ . The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res 2007; 13: 5995–6000.
Harrington KJ, Melcher AA, Bateman AR, Ahmed A, Vile RG . Cancer gene therapy: part 2. Candidate transgenes and their clinical development. Clin Oncol (R Coll Radiol) 2002; 14: 148–169.
Harrington KJ, Vile R, Pandha HS . Viral Therapy of Cancer. John Wiley & Sons Ltd: Chichester, UK. 1st edn. 2008: 404.
Portsmouth D, Hlavaty J, Renner M . Suicide genes for cancer therapy. Mol Aspects Med 2007; 28: 4–41.
Foloppe J, Kintz J, Futin N, Findeli A, Cordier P, Schlesinger Y et al. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Therapy 2008; 15: 1361–1371.
Kelly E, Russell SJ . History of oncolytic viruses: genesis to genetic engineering. Mol Ther 2007; 15: 651–659.
Fournier P, Schirrmacher V . Randomized clinical studies of anti-tumor vaccination: state of the art in 2008. Expert Rev Vaccines 2009; 8: 51–66.
Twigger K, Vidal L, White CL, De Bono JS, Bhide S, Coffey M et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res 2008; 14: 912–923.
Harrington KJ, Melcher A, Vassaux G, Pandha HS, Vile RG . Exploiting synergies between radiation and oncolytic viruses. Curr Opin Mol Ther 2008; 10: 362–370.
Kumar S, Gao L, Yeagy B, Reid T . Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther 2008; 10: 371–379.
Pandha HS, Heinemann L, Simpson GR, Melcher A, Prestwich R, Errington F et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res 2009; 15: 6158–6166.
Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med 2008; 14: 37–44.
Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G . The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Therapy 1995; 2: 107–115.
Chiocca EA . The host response to cancer virotherapy. Curr Opin Mol Ther 2008; 10: 38–45.
Galivo F, Diaz RM, Wongthida P, Thompson J, Kottke T, Barber G et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Therapy 2009; 17: 158–170.
de Wilt JH, Bout A, Eggermont AM, van Tiel ST, de Vries MW, ten Hagen TL et al. Adenovirus-mediated interleukin 3 beta gene transfer by isolated limb perfusion inhibits growth of limb sarcoma in rats. Hum Gene Ther 2001; 12: 489–502.
White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Therapy 2008; 15: 911–920.
Fujisawa M, Miyake H . Significance of micrometastases in prostate cancer. Surg Oncol 2008; 17: 247–252.
Ignatiadis M, Georgoulias V, Mavroudis D . Micrometastatic disease in breast cancer: clinical implications. Eur J Cancer 2008; 44: 2726–2736.
Grunhagen DJ, de Wilt JH, Graveland WJ, van Geel AN, Eggermont AM . The palliative value of tumor necrosis factor alpha-based isolated limb perfusion in patients with metastatic sarcoma and melanoma. Cancer 2006; 106: 156–162.
Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19: 3635–3648.
Thomas JM . Sentinel lymph node biopsy in malignant melanoma. BMJ 2008; 336: 902–903.
Creech Jr O, Krementz ET, Ryan RF, Winblad JN . Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg 1958; 148: 616–632.
Ten Hagen TL, Eggermont AMM . Isolated limb and organ perfusion laboratory models. In: Schlag PM, Stein U, Eggermont AMM (eds). Regional Cancer Therapy. vol. 1. Humana Press: Berlin, 2007. pp 29–44.
Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ . High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol 1992; 10: 52–60.
Agrawal VK, Copeland KM, Barbachano Y, Rahim A, Seth R, White CL et al. Microvascular free tissue transfer for gene delivery: in vivo evaluation of different routes of plasmid and adenoviral delivery. Gene Therapy 2009; 16: 78–92.
Eggermont AM, de Wilt JH, ten Hagen TL . Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 2003; 4: 429–437.
Benckhuijsen C, Kroon BB, van Geel AN, Wieberdink J . Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol 1988; 14: 157–163.
Marinelli A, van Dierendonck JH, van Brakel GM, Irth H, Kuppen PJ, Tjaden UR et al. Increasing the effective concentration of melphalan in experimental rat liver tumours: comparison of isolated liver perfusion and hepatic artery infusion. Br J Cancer 1991; 64: 1069–1075.
de Wilt JH, ten Hagen TL, de Boeck G, van Tiel ST, de Bruijn EA, Eggermont AM . Tumour necrosis factor alpha increases melphalan concentration in tumour tissue after isolated limb perfusion. Br J Cancer 2000; 82: 1000–1003.
van der Veen AH, de Wilt JH, Eggermont AM, van Tiel ST, Seynhaeve AL, ten Hagen TL . TNF-alpha augments intratumoural concentrations of doxorubicin in TNF-alpha-based isolated limb perfusion in rat sarcoma models and enhances anti-tumour effects. Br J Cancer 2000; 82: 973–980.
Norda A, Loos U, Sastry M, Goehl J, Hohenberger W . Pharmacokinetics of melphalan in isolated limb perfusion. Cancer Chemother Pharmacol 1999; 43: 35–42.
Koops HS, Vaglini M, Suciu S, Kroon BB, Thompson JF, Gohl J et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol 1998; 16: 2906–2912.
Kerr DJ, McArdle CS, Ledermann J, Taylor I, Sherlock DJ, Schlag PM et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet 2003; 361: 368–373.
Grunhagen DJ, de Wilt JH, van Geel AN, Eggermont AM . Isolated limb perfusion for melanoma patients––a review of its indications and the role of tumour necrosis factor-alpha. Eur J Surg Oncol 2006; 32: 371–380.
Hayes AJ, Neuhaus SJ, Clark MA, Thomas JM . Isolated limb perfusion with melphalan and tumor necrosis factor alpha for advanced melanoma and soft-tissue sarcoma. Ann Surg Oncol 2007; 14: 230–238.
Bonvalot S, Muret J, Debaere T . Pelvic perfusion for locally advanced tumors. Bull Cancer 2009; 96: 103–109.
Zeh III HJ, Brown CK, Holtzman MP, Egorin MJ, Holleran JL, Potter DM et al. A phase I study of hyperthermic isolated hepatic perfusion with oxaliplatin in the treatment of unresectable liver metastases from colorectal cancer. Ann Surg Oncol 2009; 16: 385–394.
Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 2007; 25: 837–844.
Kroon HM, Moncrieff M, Kam PC, Thompson JF . Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol 2008; 15: 3003–3013.
Beasley GM, Caudle A, Petersen RP, McMahon NS, Padussis J, Mosca PJ et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg 2009; 208: 706–715; discussion 707–715.
Hoving S, Brunstein F, aan de Wiel-Ambagtsheer G, van Tiel ST, de Boeck G, de Bruijn EA et al. Synergistic antitumor response of interleukin 2 with melphalan in isolated limb perfusion in soft tissue sarcoma-bearing rats. Cancer Res 2005; 65: 4300–4308.
Kottke T, Thompson J, Diaz RM, Pulido J, Willmon C, Coffey M et al. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin Cancer Res 2009; 15: 561–569.
Brunstein F, Eggermont AM, de Wiel-Ambagtsheer G, van Tiel ST, Rens J, ten Hagen TL . Synergistic antitumor effects of histamine plus melphalan in isolated hepatic perfusion for liver metastases. Ann Surg Oncol 2007; 14: 795–801.
Barber FD, Mavligit G, Kurzrock R . Hepatic arterial infusion chemotherapy for metastatic colorectal cancer: a concise overview. Cancer Treat Rev 2004; 30: 425–436.
van Ijken MG, van Etten B, Brunstein F, ten Hagen TL, Guetens G, de Wilt JH et al. Bio-chemotherapeutic strategies and the (dis) utility of hypoxic perfusion of liver, abdomen and pelvis using balloon catheter techniques. Eur J Surg Oncol 2005; 31: 807–816.
van Ijken MG, de Bruijn EA, de Boeck G, ten Hagen TL, van der Sijp JR, Eggermont AM . Isolated hypoxic hepatic perfusion with tumor necrosis factor-alpha, melphalan, and mitomycin C using balloon catheter techniques: a pharmacokinetic study in pigs. Ann Surg 1998; 228: 763–770.
Sugarbaker PH . Peritonectomy procedures. Ann Surg 1995; 221: 29–42.
Shen P, Stewart IV JH, Levine EA . Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: non-colorectal indications. Curr Probl Cancer 2009; 33: 168–193.
Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996; 335: 1950–1955.
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001; 19: 1001–1007.
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34–43.
Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009; 27: 6237–6242.
Chua TC, Yan TD, Saxena A, Morris DL . Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann Surg 2009; 249: 900–907.
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737–3743.
Rainov NG . A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 2000; 11: 2389–2401.
Kemeny N, Brown K, Covey A, Kim T, Bhargava A, Brody L et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther 2006; 17: 1214–1224.
Reid T, Galanis E, Abbruzzese J, Sze D, Andrews J, Romel L et al. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Therapy 2001; 8: 1618–1626.
Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res 2002; 62: 6070–6079.
Atencio IA, Grace M, Bordens R, Fritz M, Horowitz JA, Hutchins B et al. Biological activities of a recombinant adenovirus p53 (SCH 58500) administered by hepatic arterial infusion in a Phase 1 colorectal cancer trial. Cancer Gene Ther 2006; 13: 169–181.
Tian G, Liu J, Zhou JS, Chen W . Multiple hepatic arterial injections of recombinant adenovirus p53 and 5-fluorouracil after transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a pilot phase II trial. Anticancer Drugs 2009; 20: 389–395.
Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol 2002; 20: 1562–1569.
Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res 2010; 70: 875–882.
Carroll NM, Alexander Jr HR . Isolation perfusion of the liver. Cancer J 2002; 8: 181–193.
Henry SD, van der Wegen P, Metselaar HJ, Scholte BJ, Tilanus HW, van der Laan LJ . Hydroxyethyl starch-based preservation solutions enhance gene therapy vector delivery under hypothermic conditions. Liver Transpl 2008; 14: 1708–1717.
Baker AH, McVey JH, Waddington SN, Di Paolo NC, Shayakhmetov DM . The influence of blood on in vivo adenovirus bio-distribution and transduction. Mol Ther 2007; 15: 1410–1416.
Oi K, Davies WR, Tazelaar HD, Bailey KR, Federspiel MJ, Russell SJ et al. Ex vivo hypothermic recirculatory adenoviral gene transfer to the transplanted pig heart. J Gene Med 2006; 8: 795–803.
Persson R, Wohlfart C, Svensson U, Everitt E . Virus-receptor interaction in the adenovirus system: characterization of the positive cooperative binding of virions on HeLa cells. J Virol 1985; 54: 92–97.
Chang E, Chalikonda S, Friedl J, Xu H, Phan GQ, Marincola FM et al. Targeting vaccinia to solid tumors with local hyperthermia. Hum Gene Ther 2005; 16: 435–444.
Fujita S, Eguchi A, Okabe J, Harada A, Sasaki K, Ogiwara N et al. Sendai virus-mediated gene delivery into hepatocytes via isolated hepatic perfusion. Biol Pharm Bull 2006; 29: 1728–1734.
Kroon BB . Regional isolation perfusion in melanoma of the limbs; accomplishments, unsolved problems, future. Eur J Surg Oncol 1988; 14: 101–110.
O'Donnell JM, Lewandowski ED . Efficient, cardiac-specific adenoviral gene transfer in rat heart by isolated retrograde perfusion in vivo. Gene Therapy 2005; 12: 958–964.
Reid T, Warren R, Kirn D . Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther 2002; 9: 979–986.
Wiley . Gene Therapy Clinical Trials Worldwide. John Wiley and Sons Inc.: Chichester, UK, 2009.
Elaraj DM, Alexander HR . Current role of hepatic artery infusion and isolated liver perfusion for the treatment of colorectal cancer liver metastases. Cancer J 2004; 10: 128–138.
de Roos WK, Fallaux FJ, Marinelli AW, Lazaris-Karatzas A, von Geusau AB, van der Eb MM et al. Isolated-organ perfusion for local gene delivery: efficient adenovirus-mediated gene transfer into the liver. Gene Therapy 1997; 4: 55–62.
Nomura N, Kasuya H, Watanabe I, Shikano T, Shirota T, Misawa M et al. Considerations for intravascular administration of oncolytic herpes virus for the treatment of multiple liver metastases. Cancer Chemother Pharmacol 2009; 63: 321–330.
Hiraoka K, Kimura T, Logg CR, Kasahara N . Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin Cancer Res 2006; 12: 7108–7116.
Hiraoka K, Kimura T, Logg CR, Tai CK, Haga K, Lawson GW et al. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res 2007; 67: 5345–5353.
van Etten B, ten Hagen TL, de Vries MR, Ambagtsheer G, Huet T, Eggermont AM . Prerequisites for effective adenovirus mediated gene therapy of colorectal liver metastases in the rat using an intracellular neutralizing antibody fragment to p21-Ras. Br J Cancer 2002; 86: 436–442.
Cronin CG, Lohan DG, O'Riordan C, Gough N, O'Sullivan GJ . Isolated limb infusion: technique description and clinical application. J Vasc Interv Radiol 2009; 20: 837–841.
Thompson JF, Kam PC . Isolated limb infusion for melanoma: a simple but effective alternative to isolated limb perfusion. J Surg Oncol 2004; 88: 1–3.
Testori A, Rutkowski P, Marsden J, Bastholt L, Chiarion-Sileni V, Hauschild A et al. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann Oncol 2009; 20 (Suppl 6): vi22–vi29.
Milas M, Feig B, Yu D, Oriuchi N, Cromeens D, Ge T et al. Isolated limb perfusion in the sarcoma-bearing rat: a novel preclinical gene delivery system. Clin Cancer Res 1997; 3: 2197–2203.
Manusama ER, Nooijen PT, Stavast J, Durante NM, Marquet RL, Eggermont AM . Synergistic antitumour effect of recombinant human tumour necrosis factor alpha with melphalan in isolated limb perfusion in the rat. Br J Surg 1996; 83: 551–555.
Bauer TW, Gutierrez M, Dudrick DJ, Li J, Blair IA, Menon C et al. A human melanoma xenograft in a nude rat responds to isolated limb perfusion with TNF plus melphalan. Surgery 2003; 133: 420–428.
Hannay J, Davis JJ, Yu D, Liu J, Fang B, Pollock RE et al. Isolated limb perfusion: a novel delivery system for wild-type p53 and fiber-modified oncolytic adenoviruses to extremity sarcoma. Gene Therapy 2007; 14: 671–681.
Van Etten B, Van Tiel ST, Ambagtsheer G, Eggermont AM, Ten Hagen TL . Isolated limb perfusion based anti-p21ras gene therapy in a rat rhabdomyosarcoma. Anticancer Res 2004; 24: 2295–2301.
de Roos WK, de Wilt JH, van Der Kaaden ME, Manusama ER, de Vries MW, Bout A et al. Isolated limb perfusion for local gene delivery: efficient and targeted adenovirus-mediated gene transfer into soft tissue sarcomas. Ann Surg 2000; 232: 814–821.
Naik AM, Chalikonda S, McCart JA, Xu H, Guo ZS, Langham G et al. Intravenous and isolated limb perfusion delivery of wild type and a tumor-selective replicating mutant vaccinia virus in nonhuman primates. Hum Gene Ther 2006; 17: 31–45.
Richter M, Iwata A, Nyhuis J, Nitta Y, Miller AD, Halbert CL et al. Adeno-associated virus vector transduction of vascular smooth muscle cells in vivo. Physiol Genomics 2000; 2: 117–127.
Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther 2007; 15: 1686–1693.
Brooks AD, Ng B, Liu D, Brownlee M, Burt M, Federoff HJ et al. Specific organ gene transfer in vivo by regional organ perfusion with herpes viral amplicon vectors: implications for local gene therapy. Surgery 2001; 129: 324–334.
Acknowledgements
TP is supported by a research grant from the Royal College of Surgeons of England (Lincoln's Inn Fields, London, UK). RS is supported by a research grant from the Royal Colleges of Surgeons of Edinburgh and Ireland (Nicolson Street, Edinburgh, UK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pencavel, T., Seth, R., Hayes, A. et al. Locoregional intravascular viral therapy of cancer: precision guidance for Paris's arrow?. Gene Ther 17, 949–960 (2010). https://doi.org/10.1038/gt.2010.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.48
Keywords
This article is cited by
-
Optimizing oncolytic virotherapy in cancer treatment
Nature Reviews Drug Discovery (2019)