Abstract
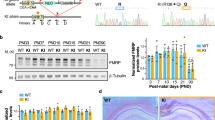
Fragile X syndrome (FXS) is caused by a mutation that silences the fragile X mental retardation gene (FMR1), which encodes the fragile X mental retardation protein (FMRP). To determine whether FMRP replacement can rescue phenotypic deficits in a fmr1-knockout (KO) mouse model of FXS, we constructed an adeno-associated virus-based viral vector that expresses the major central nervous system (CNS) isoform of FMRP. Using this vector, we tested whether FMRP replacement could rescue the fmr1-KO phenotype of enhanced long-term depression (LTD), a form of synaptic plasticity that may be linked to cognitive impairments associated with FXS. Extracellular excitatory postsynaptic field potentials were recorded from CA3–CA1 synaptic contacts in hippocampal slices from wild-type (WT) and fmr1-KO mice in the presence of AP-5 and anisomycin. Paired-pulse low-frequency stimulation (PP-LFS)-induced LTD is enhanced in slices obtained from fmr1 KO compared with WT mice. Analyses of hippocampal synaptic function in fmr1-KO mice that received hippocampal injections of vector showed that the PP-LFS-induced LTD was restored to WT levels. These results indicate that expression of the major CNS isoform of FMRP alone is sufficient to rescue this phenotype and suggest that post-developmental protein replacement may have the potential to improve cognitive function in FXS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 1991; 66: 817–822.
Verheij C, Bakker CE, de Graaff E, Keulemans J, Willemsen R, Verkerk AJ et al. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature 1993; 363: 722–724.
Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991; 65: 905–914.
Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 1991; 252: 1097–1102.
O’Donnell WT, Warren ST . A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci 2002; 25: 315–338.
Huber KM, Gallagher SM, Warren ST, Bear MF . Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA 2002; 99: 7746–7750.
Nosyreva ED, Huber KM . Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol 2006; 95: 3291–3295.
Bliss TV, Collingridge GL . A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993; 361: 31–39.
Mulkey RM, Malenka RC . Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 1992; 9: 967–975.
Pfeiffer BE, Huber KM . Current advances in local protein synthesis and synaptic plasticity. J Neurosci 2006; 26: 7147–7150.
Sutton MA, Schuman EM . Local translational control in dendrites and its role in long-term synaptic plasticity. J Neurobiol 2005; 64: 116–131.
Ashley Jr CT, Wilkinson KD, Reines D, Warren ST . FMR1 protein: conserved RNP family domains and selective RNA binding. Science 1993; 262: 563–566.
Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM et al. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci 1997; 17: 1539–1547.
Kooy RF, Willemsen R, Oostra BA . Fragile X syndrome at the turn of the century. Mol Med Today 2000; 6: 193–198.
The Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell 1994; 78: 23–33.
Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA 1997; 94: 5395–5400.
Burger C, Nash K, Mandel RJ . Recombinant adeno-associated viral vectors in the nervous system. Hum Gene Ther 2005; 16: 781–791.
Peden CS, Burger C, Muzyczka N, Mandel RJ . Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol 2004; 78: 6344–6359.
Mandel RJ, Manfredsson FP, Foust KD, Rising A, Reimsnider S, Nash K et al. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol Ther 2006; 13: 463–483.
Xu L, Daly T, Gao C, Flotte TR, Song S, Byrne BJ et al. CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum Gene Ther 2001; 12: 563–573.
Doll RF, Crandall JE, Dyer CA, Aucoin JM, Smith F . Comparison of promoter strengths on gene delivery into mammalian brain cells using AAV vectors. Gene Therapy 1996; 3: 437–447.
Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2 and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 2004; 10: 302–317.
Bear MF, Huber KM, Warren ST . The mGluR theory of fragile X mental retardation. Trends Neurosci 2004; 27: 370–377.
Nosyreva ED, Huber KM . Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J Neurosci 2005; 25: 2992–3001.
Musumeci SA, Calabrese G, Bonaccorso CM, D’Antoni S, Brouwer JR, Bakker CE et al. Audiogenic seizure susceptibility is reduced in fragile X knockout mice after introduction of FMR1 transgenes. Exp Neurol 2007; 203: 233–240.
Brown V, Small K, Lakkis L, Feng Y, Gunter C, Wilkinson KD et al. Purified recombinant Fmrp exhibits selective RNA binding as an intrinsic property of the fragile X mental retardation protein. J Biol Chem 1998; 273: 15521–15527.
Lo WD, Qu G, Sferra TJ, Clark R, Chen R, Johnson PR et al. Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum Gene Ther 1999; 10: 201–213.
Rattazzi MC, LaFauci G, Brown WT . Prospects for gene therapy in the fragile X syndrome. Ment Retard Dev Disabil Res Rev 2004; 10: 75–81.
Gantois I, Bakker CE, Reyniers E, Willemsen R, D’Hooge R, De Deyn PP et al. Restoring the phenotype of fragile X syndrome: insight from the mouse model. Curr Mol Med 2001; 1: 447–455.
Huber KM, Roder JC, Bear MF . Chemical induction of mGluR5- and protein synthesis—dependent long-term depression in hippocampal area CA1. J Neurophysiol 2001; 86: 321–325.
Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E . Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 2006; 51: 441–454.
Peier AM, McIlwain KL, Kenneson A, Warren ST, Paylor R, Nelson DL . (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet 2000; 9: 1145–1159.
Denman RB, Sung YJ . Species-specific and isoform-specific RNA binding of human and mouse fragile X mental retardation proteins. Biochem Biophys Res Commun 2002; 292: 1063–1069.
Xu R, Janson CG, Mastakov M, Lawlor P, Young D, Mouravlev A . Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Therapy 2001; 8: 1323–1332.
Graham FL, Smiley J, Russell WC, Nairn R . Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 1977; 36: 59–74.
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Therapy 1999; 6: 973–985.
Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites Jr TJ et al. Production and purification of serotype 1, 2 and 5 recombinant adeno-associated viral vectors. Methods 2002; 28: 158–167.
Acknowledgements
This work was supported by National Institutes of Health Grants AG14979 and MH059891, (TCF), the Evelyn F. McKnight Brain Research Grant (TCF), a University of Florida Alumni Fellowship (KB), a grant from the FRAXA Research Foundation (DCB), and an Investigator in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund (DCB). Special thanks to Marda Jorgensen for contributing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeier, Z., Kumar, A., Bodhinathan, K. et al. Fragile X mental retardation protein replacement restores hippocampal synaptic function in a mouse model of fragile X syndrome. Gene Ther 16, 1122–1129 (2009). https://doi.org/10.1038/gt.2009.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.83
Keywords
This article is cited by
-
Postnatal GABAA Receptor Activation Alters Synaptic Plasticity and Cognition in Adult Wistar Rats
Molecular Neurobiology (2022)
-
The role of GABAergic signalling in neurodevelopmental disorders
Nature Reviews Neuroscience (2021)
-
Developmental studies in fragile X syndrome
Journal of Neurodevelopmental Disorders (2020)
-
Reactivation of FMR1 gene expression is a promising strategy for fragile X syndrome therapy
Gene Therapy (2020)
-
Autism spectrum disorder: prospects for treatment using gene therapy
Molecular Autism (2018)