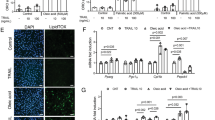
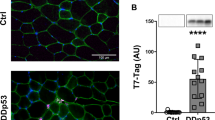
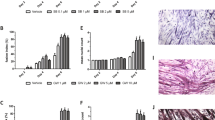
Abstract
Loss of muscle protein is a serious complication of catabolic diseases and contributes substantially to patients' morbidity and mortality. This muscle loss is mediated largely by the activation of the ubiquitin–proteasome system; however, caspase-3 catalyzes an initial step in this process by cleaving actomyosin into small protein fragments that are rapidly degraded by the proteasome-dependent proteolytic pathway. We hypothesized that X-chromosome linked inhibitor of apoptosis protein (XIAP), an endogenous caspase-3 inhibitor, would block this first step in the cleavage of actomyosin that would make XIAP a candidate for treating muscle wasting. To determine if XIAP could attenuate muscle protein degradation, we used a recombinant lentivirus (Len-XIAP) encoding the full-length human XIAP cDNA to express XIAP in vivo. In muscle of streptozotocin-treated insulin-deficient mice, total muscle protein degradation, caspase-3 activity, and myofibril destruction were increased while XIAP was decreased. Overexpression of XIAP in these mice attenuated the excessive muscle protein degradation. Increased proteasome activity, caspase-3 activity and myofibril protein breakdown were all reduced. The ability of XIAP to prevent the loss of muscle protein suggests that XIAP could be a therapeutic reagent for muscle atrophy in catabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Abbreviations
- EDL:
-
extensor digitorum longus
- MuLV:
-
murine leukemia virus
- STZ:
-
streptozotocin
- XIAP:
-
X-chromosome linked inhibitor of apoptosis protein.
References
Griffiths RD . Muscle mass, survival, and the elderly ICU patient. Nutrition 1996; 12: 456–458.
Windsor JA, Hill GL . Risk factors for postoperative pneumonia. The importance of protein depletion. Ann Surg 1988; 208: 209–214.
Siu PM, Alway SE . Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 2005; 565: 309–323.
Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE . Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1288–R1296.
Siu PM, Pistilli EE, Butler DC, Alway SE . Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol 2005; 288: C338–349.
Galban VD, Evangelista EA, Migliorini RH, do Carmo Kettelhut I . Role of ubiquitin-proteasome-dependent proteolytic process in degradation of muscle protein from diabetic rabbits. Mol Cell Biochem 2001; 225: 35–41.
Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J . Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest 1995; 95: 2926–2937.
Bailey JL, Wang X, England BK, Price SR, Mitch WE . The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest 1996; 97: 1447–1453.
Mansoor O, Beaufrere B, Boirie Y, Ralliere C, Taillandier D, Aurousseau E et al. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci USA 1996; 93: 2714–2718.
Fang CH, Tiao G, James H, Ogle C, Fischer JE, Hasselgren PO . Burn injury stimulates multiple proteolytic pathways in skeletal muscle, including the ubiquitin-energy-dependent pathway. J Am Coll Surg 1995; 180: 161–170.
Vescovo G, Volterrani M, Zennaro R, Sandri M, Ceconi C, Lorusso R et al. Apoptosis in the skeletal muscle of patients with heart failure: investigation of clinical and biochemical changes. Heart 2000; 84: 431–437.
Libera LD, Zennaro R, Sandri M, Ambrosio GB, Vescovo G . Apoptosis and atrophy in rat slow skeletal muscles in chronic heart failure. Am J Physiol 1999; 277: C982–C986.
Price SR, Bailey JL, Wang X, Jurkovitz C, England BK, Ding X et al. Muscle wasting in insulinopenic rats results from activation of the ATP-dependent, ubiquitin–proteasome proteolytic pathway by a mechanism including gene transcription. J Clin Invest 1996; 98: 1703–1708.
Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR . Evaluation of signals activating ubiquitin–proteasome proteolysis in a model of muscle wasting. Am J Physiol 1999; 276: C1132–1138.
Solomon V, Goldberg AL . Importance of the ATP-ubiquitin–proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 1996; 271: 26690–26697.
Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 2004; 113: 115–123.
Deveraux QL, Reed JC . IAP family proteins – suppressors of apoptosis. Genes Dev 1999; 13: 239–252.
Holcik M, Korneluk RG . XIAP, the guardian angel. Nat Rev Mol Cell Biol 2001; 2: 550–556.
Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 1998; 17: 2215–2223.
Deveraux QL, Takahashi R, Salvesen GS, Reed JC . X-linked IAP is a direct inhibitor of cell-death proteases. Nature 1997; 388: 300–304.
Xu D, Bureau Y, McIntyre DC, Nicholson DW, Liston P, Zhu Y et al. Attenuation of ischemia-induced cellular and behavioral deficits by X chromosome-linked inhibitor of apoptosis protein overexpression in the rat hippocampus. J Neurosci 1999; 19: 5026–5033.
Case SS, Price MA, Jordan CT, Yu XJ, Wang L, Bauer G et al. Stable transduction of quiescent CD34(+)CD38(−) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA 1999; 96: 2988–2993.
Kugler S, Straten G, Kreppel F, Isenmann S, Liston P, Bahr M . The X-linked inhibitor of apoptosis (XIAP) prevents cell death in axotomized CNS neurons in vivo. Cell Death Differ 2000; 7: 815–824.
Liu X, Kim CN, Yang J, Jemmerson R, Wang X . Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 1996; 86: 147–157.
Larochelle A, Peng KW, Russell SJ . Lentiviral vector targeting. Curr Top Microbiol Immunol 2002; 261: 143–163.
Hunter RB, Kandarian SC . Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest 2004; 114: 1504–1511.
Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE . Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin–proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 2004; 15: 1537–1545.
Marinovic AC, Zheng B, Mitch WE, Price SR . Ubiquitin (UbC) expression in muscle cells is increased by glucocorticoids through a mechanism involving Sp1 and MEK1. J Biol Chem 2002; 277: 16673–16681.
Du J, Mitch WE, Wang X, Price SR . Glucocorticoids induce proteasome C3 subunit expression in L6 muscle cells by opposing the suppression of its transcription by NF-kappa B. J Biol Chem 2000; 275: 19661–19666.
Yasuhara S, Perez ME, Kanakubo E, Yasuhara Y, Shin YS, Kaneki M et al. Skeletal muscle apoptosis after burns is associated with activation of proapoptotic signals. Am J Physiol Endocrinol Metab 2000; 279: E1114–1121.
Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V et al. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 1997; 273: C579–C587.
Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC et al. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 1999; 18: 5242–5251.
Wang XL, Zhang L, Youker K, Zhang MX, Wang J, Lemaire SA et al. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes 2006; 55: 2301–2310.
Roy N, Mahadevan MS, McLean M, Shutler G, Yaraghi Z, Farahani R et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 1995; 80: 167–178.
Trono D . HIV-based vectors: getting the best out of the worst. J Gene Med 2000; 2: 61–63.
Trono D . Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Therapy 2000; 7: 20–23.
MacKenzie TC, Kobinger GP, Kootstra NA, Radu A, Sena-Esteves M, Bouchard S et al. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol Therapy 2002; 6: 349–358.
Kobinger GP, Louboutin JP, Barton ER, Sweeney HL, Wilson JM . Correction of the dystrophic phenotype by in vivo targeting of muscle progenitor cells. Hum Gene Therapy 2003; 14: 1441–1449.
Riviere C, Danos O, Douar AM . Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Therapy 2006; 13: 1300–1308.
Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D . Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 1997; 15: 871–875.
Seppen J, van Til NP, van der Rijt R, Hiralall JK, Kunne C, Elferink RP . Immune response to lentiviral bilirubin UDP-glucuronosyltransferase gene transfer in fetal and neonatal rats. Gene Therapy 2006; 13: 672–677.
Clark AS, Mitch WE . Comparison of protein synthesis and degradation in incubated and perfused muscle. Biochem J 1983; 212: 649–653.
Wang X, Hu Z, Hu J, Du J, Mitch WE . Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin–proteasome pathway by defects in muscle cell signaling. Endocrinology 2006; 147: 4160–4168.
Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD . Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 2000; 288: 874–877.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Kim D, Sands JM, Klein JD . Changes in renal medullary transport proteins during uncontrolled diabetes mellitus in rats. Am J Physiol Renal Physiol 2003; 285: F303–F309.
Acknowledgements
We thank Dr Didier Trono for providing the packaging plasmid; Drs James Wilson and Gary Koringer for providing the five lentivirus envelope plasmids; Dr Yili Yang for the pEBB.XIAP plasmid, Dr Joe M LeDoux for pCCL.CMV.GFP plasmid. We thank Dr Russ Price and Dr James Bailey for their critical reading of this manuscript. This study was supported by a Norman S Coplon Extramural Research Grant from Satellite Health, an American Diabetes Association Junior Faculty Award 1-04-JF-48 and NIH R21 Grant DK62796 to XHW, and NIH R01 DK062081 to JDK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Hu, J., Du, J. et al. X-chromosome linked inhibitor of apoptosis protein inhibits muscle proteolysis in insulin-deficient mice. Gene Ther 14, 711–720 (2007). https://doi.org/10.1038/sj.gt.3302927
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302927
Keywords
This article is cited by
-
Mitochondrial Biogenesis and Fragmentation as Regulators of Muscle Protein Degradation
Current Hypertension Reports (2010)