Abstract
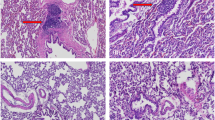
Isolated limb perfusion (ILP) is a limb salvage surgical modality used to deliver chemotherapy and biologic agents to locally advanced and recurrent extremity soft tissue sarcoma (STS), and may be readily tailored for delivery of gene therapy. We set out to test the feasibility of delivering AdFLAGp53 (replication incompetent adenovirus bearing FLAG-tagged wild-type p53) and Ad.hTC.GFP/E1a.RGD (a fiber-modified, replication selective oncolytic adenovirus) into human leiomyosarcoma xenografts by ILP. Nude rats bearing SKLMS-1 tumors in their hind limbs underwent ILP with escalating doses of AdLacZ or AdFLAGp53 (study 1), or with Ad.CMV.GFP.RGD or Ad.hTC.GFP/E1a.RGD (study 2) following in vitro confirmation of therapeutic potential in STS cell lines and strains. Seventy-two hours after delivery, reverse transcription-polymerase chain reaction confirmed FLAGp53 expression, and immunohistochemistry confirmed diffuse upregulation of p21CIP1/WAF1 in ILP-treated tumors. Ad.hTC.GFP/E1a.RGD perfused tumors demonstrated robust macroscopic transgene expression throughout their substance, but not in perfused normal tissues, 21 days after delivery. Intra-tumoral viral replication was confirmed by immunohistochemical staining for early (E1a) and late (hexon) viral protein expression. Terminal deoxynucleotidyl transferase-mediated-digoxigenin nick end-labeling staining identified foci of cell death within regions of viral replication. In conclusion, therapeutic adenoviral gene therapy against limb borne human STS can be successfully delivered by ILP and warrants further investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C et al. Cancer statistics. CA Cancer J Clin 2006; 56: 106–130.
Gaynor JJ, Tan CC, Casper ES, Collin CF, Friedrich C, Shiu M et al. Refinement of clinicopathologic staging for localized soft tissue sarcoma of the extremity: a study of 423 adults. J Clin Oncol 1992; 10: 1317–1329.
Kane III JM . Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Curr Opin Oncol 2004; 16: 328–332.
Lewis JJ, Leung D, Casper ES, Woodruff J, Hajdu SI, Brennan MF . Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg 1999; 134: 190–194.
Grunhagen DJ, de Wilt JH, Graveland WJ, Verhoef C, van Geel AN, Eggermont AM . Outcome and prognostic factor analysis of 217 consecutive isolated limb perfusions with tumor necrosis factor-alpha and melphalan for limb-threatening soft tissue sarcoma. Cancer 2006; 106: 1776–1784.
Noorda EM, Vrouenraets BC, Nieweg OE, Van Coevorden F, Kroon BB . Isolated limb perfusion: what is the evidence for its use? Ann Surg Oncol 2004; 11: 837–845.
Feig BW . Isolated limb perfusion for extremity sarcoma. Curr Oncol Rep 2000; 2: 491–494.
Milas M, Feig B, Yu D, Oriuchi N, Cromeens D, Ge T et al. Isolated limb perfusion in the sarcoma-bearing rat: a novel preclinical gene delivery system. Clin Cancer Res 1997; 3: 2197–2203.
Mocellin S, Rossi CR, Brandes A, Nitti D . Adult soft tissue sarcomas: conventional therapies and molecularly targeted approaches. Cancer Treat Rev 2006; 32: 9–27.
de Wilt JH, Bout A, Eggermont AM, van Tiel ST, de Vries MW, ten Hagen TL et al. Adenovirus-mediated interleukin 3 beta gene transfer by isolated limb perfusion inhibits growth of limb sarcoma in rats. Hum Gene Ther 2001; 12: 489–502.
Eggermont AM, de Wilt JH, ten Hagen TL . Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 2003; 4: 429–437.
de Roos WK, de Wilt JH, van Der Kaaden ME, Manusama ER, de Vries MW, Bout A et al. Isolated limb perfusion for local gene delivery: efficient and targeted adenovirus-mediated gene transfer into soft tissue sarcomas. Ann Surg 2000; 232: 814–821.
Van Etten B, Van Tiel ST, Ambagtsheer G, Eggermont AM, Ten Hagen TL . Isolated limb perfusion based anti-p21ras gene therapy in a rat rhabdomyosarcoma. Anticancer Res 2004; 24: 2295–2301.
Pollock R, Lang A, Ge T, Sun D, Tan M, Yu D . Wild-type p53 and a p53 temperature-sensitive mutant suppress human soft tissue sarcoma by enhancing cell cycle control. Clin Cancer Res 1998; 4: 1985–1994.
Milas M, Yu D, Lang A, Ge T, Feig B, El-Naggar AK et al. Adenovirus-mediated p53 gene therapy inhibits human sarcoma tumorigenicity. Cancer Gene Ther 2000; 7: 422–429.
Zhan M, Yu D, Lang A, Li L, Pollock RE . Wild type p53 sensitizes soft tissue sarcoma cells to doxorubicin by down-regulating multidrug resistance-1 expression. Cancer 2001; 92: 1556–1566.
Zhang L, Yu D, Hu M, Xiong S, Lang A, Ellis LM et al. Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res 2000; 60: 3655–3661.
Swisher SG, Roth JA, Nemunaitis J, Lawrence DD, Kemp BL, Carrasco CH et al. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J Natl Cancer Inst 1999; 91: 763–771.
Weill D, Mack M, Roth J, Swisher S, Proksch S, Merritt J et al. Adenoviral-mediated p53 gene transfer to non-small cell lung cancer through endobronchial injection. Chest 2000; 118: 966–970.
Warren RS, Kirn DH . Liver-directed viral therapy for cancer p53-targeted adenoviruses and beyond. Surg Oncol Clin North Am 2002; 11: 571–588.
Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol 2003; 21: 2508–2518.
Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A . Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol 2005; 79: 7478–7491.
Yotnda P, Zompeta C, Heslop HE, Andreeff M, Brenner MK, Marini F . Comparison of the efficiency of transduction of leukemic cells by fiber-modified adenoviruses. Hum Gene Ther 2004; 15: 1229–1242.
Davis JJ, Wang L, Dong F, Zhang L, Guo W, Teraishi F et al. Oncolysis and suppression of tumor growth by a GFP-expressing oncolytic adenovirus controlled by an hTERT and CMV hybrid promoter. Cancer Gene Ther 2006; 13: 720–723.
Schmitz M, Graf C, Gut T, Sirena D, Peter I, Dummer R et al. Melanoma cultures show different susceptibility towards E1A-, E1B-19 kDa- and fiber-modified replication-competent adenoviruses. Gene Ther 2006; 13: 893–905.
Witlox AM, Van Beusechem VW, Molenaar B, Bras H, Schaap GR, Alemany R et al. Conditionally replicative adenovirus with tropism expanded towards integrins inhibits osteosarcoma tumor growth in vitro and in vivo. Clin Cancer Res 2004; 10: 61–67.
Hieken TJ, Das Gupta TK . Mutant p53 expression: a marker of diminished survival in well-differentiated soft tissue sarcoma. Clin Cancer Res 1996; 2: 1391–1395.
Taubert H, Meye A, Wurl P . Prognosis is correlated with p53 mutation type for soft tissue sarcoma patients. Cancer Res 1996; 56: 4134–4136.
Toguchida J, Yamaguchi T, Ritchie B, Beauchamp RL, Dayton SH, Herrera GE et al. Mutation spectrum of the p53 gene in bone and soft tissue sarcomas. Cancer Res 1992; 52: 6194–6199.
Pollock RE, Lang A, Luo J, El-Naggar AK, Yu D . Soft tissue sarcoma metastasis from clonal expansion of p53 mutated tumor cells. Oncogene 1996; 12: 2035–2039.
McNeish IA, Bell SJ, Lemoine NR . Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Therapy 2004; 11: 497–503.
Zeimet AG, Marth C . Why did p53 gene therapy fail in ovarian cancer? Lancet Oncol 2003; 4: 415–422.
Fang B, Roth JA . The role of gene therapy in combined modality treatment strategies for cancer. Curr Opin Mol Ther 2003; 5: 475–482.
Freytag SO, Kim JH, Brown SL, Barton K, Lu M, Chung M . Gene therapy strategies to improve the effectiveness of cancer radiotherapy. Expert Opin Biol Ther 2004; 4: 1757–1770.
Kirn D, Martuza RL, Zwiebel J . Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med 2001; 7: 781–787.
Hu M, Nicolson GL, Trent II JC, Yu D, Zhang L, Lang A et al. Characterization of 11 human sarcoma cell strains: evaluation of cytogenetics, tumorigenicity, metastasis, and production of angiogenic factors. Cancer 2002; 95: 1569–1576.
Acknowledgements
This work was supported in part by the MD Anderson Cancer Center Core Grant P30-CA16672 from the National Cancer Institute, and NIH RO1 CA 67802 (to REP), CA 109570 (to DY) and CA 098582-01A1 (to BF). We thank Peggy Tinkey and Gary Klaassen, Department of Veterinary Medicine and Surgery, MD Anderson Cancer Center for expert assistance with refinement of the ILP model; and Carol M Johnston for immunohistochemistry technical advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt).
Rights and permissions
About this article
Cite this article
Hannay, J., Davis, J., Yu, D. et al. Isolated limb perfusion: a novel delivery system for wild-type p53 and fiber-modified oncolytic adenoviruses to extremity sarcoma. Gene Ther 14, 671–681 (2007). https://doi.org/10.1038/sj.gt.3302911
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302911
Keywords
This article is cited by
-
Locoregional intravascular viral therapy of cancer: precision guidance for Paris's arrow?
Gene Therapy (2010)
-
In vivo comparison of transduction efficiency with recombinant adenovirus-mediated p53 in a human colon cancer mouse model by different delivery routes
The Chinese-German Journal of Clinical Oncology (2008)