Abstract
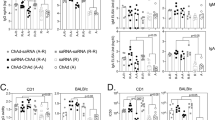
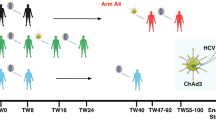
Efficient vaccination against viral agents requires a strong T-cell-mediated immune response to clear viral-infected cells. Optimal vaccination can be achieved by administration of recombinant viral vectors encoding phatogen antigens. Adenoviral vectors have attracted considerable attention as potential viral vectors for genetic vaccination owing to their favorable safety profile and potent transduction efficiency following intramuscular injection. However, the neutralizing antibody response against adenoviral capsid proteins following adenoviral vectors injection limits the success of vaccination protocols based on multiple administrations of the same adenoviral serotype. In this work, we describe efficient immunization of rhesus macaques, the preferred model for preclinical assessment, with an HCV candidate vaccine by heterologous priming–boosting with adenoviral vectors based on different serotypes. The induced responses are broad and show significant cross-strain reactivity. Boosting can be delayed for over 2 years after priming, indicating that there is long-term maintenance of resting memory cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Shiver JW, Emini AE . Recent advances in the development of HIV-1 vaccines using replication-incompentent adenovirus vectors. Annu Rev Med 2004; 55: 355–372.
Prevec L, Shneider M, Rosenthal KL, Belbeck LW, Derbyshire JB, Grahm FL . Use of human adenovirus-based vectors for antigen expression in animals. J Gen Virol 1989; 70: 429–434.
Prevec L, Campbell JB, Christie BS, Belbeck LW, Grahm FL . A recombinant human adenovirus vaccine against rabies. J Infect Dis 1990; 161: 27–30.
Rodrigues EG, Zavala F, Eichinger D, Wilson JM, Tsuji M . Single immunization dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol 1997; 158: 1268–1274.
Rocha CD, Caetano BC, Machado AV, Bruna-Romero O . Recombinant viruses as tools to induce protective cellular immunity against infectious diseases. Int Microbiol 2004; 7: 83–94.
Tatsis N, Ertl HC . Adenoviruses as vaccine vectors. Mol Ther 2004; 4: 616–629.
Casimiro DR, Bett AJ, Fu TM, Davies ME, Tang A, Wilson KA et al. Heterologous human immunodeficiency virus type 1 priming-boosting immunizationstrategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol 2004; 78: 11434–11438.
Capone S, Meola A, Ercole BB, Vitelli A, Pezzanera M, Ruggeri L et al. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-ad5 immunity and induces potent and broad cellular immune responses in rhesus macaques. J Virol 2006; 80: 1688–1699.
Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, Ertl HC . Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol 2003; 171: 6774–6779.
Roy S, Gao G, Lu Y, Zhou X, Lock M, Calcedo R et al. Characterization of a family of chimpanzee adenoviruses and development of molecular clones for gene transfer vectors. Hum Gene Ther 2004; 5: 519–530.
Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 2004; 8: 1213–1216.
Vogels RD, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 2003; 15: 8263–8271.
Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci USA 2005; 13: 4836–4841.
Evans RK, Nawrocki DK, Isopi LA, Williams DM, Casimiro DR, Chin S et al. Development of stable liquid formulations for adenovirus-based vaccines. J Pharm Sci 2004; 93: 2458–2475.
Reyes-Sandoval A, Fitzgerald JC, Grant R, Roy S, Xiang ZQ, Li Y et al. Human immunodeficiency virus type 1-specific immune response in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J Virol 2004; 78: 7392–7399.
Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 1998; 13: 1909–1917.
Rochlitz CF . Gene therapy of cancer. Drugs Today (Barc) 2000; 9: 619–629.
Rochlitz CF . Gene therapy of cancer. Swiss Med Wkly 2001; 12: 4–9.
Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM et al. An effective AIDS vaccine based on live attenuated vescicolar stomatitis virus recombinants. Cell 2001; 106: 539–549.
Folgori A, Spada E, Pezzanera M, Ruggeri L, Mele A, Garbuglia AR et al. Early impairment of HCV-specific T-cell proliferation during acute infection leads to failure of viral clearance. Gut 2006; 16: [E-pub ahead of print].
Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectros expressing a human immunodeficiency virus type 1 gag gene. J Virol 2003; 77: 6305–6313.
Spada E, Mele A, Berton A, Ruggeri L, Ferrigno L, Garbuglia AR et al. Multi-specific T-cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut 2004; 11: 1673–1681.
Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY et al. Analysis of a successful immune response against hepatitis C virus. Immunity 1999; 10: 439–449.
Evans RK, Nawrocki DK, Isopi LA, Williams DM, Casimiro DR, Chin S et al. Development of stable liquid formulations for adenovirus-based vaccines. J Pharm Sci 2004; 93: 2458–2475.
Catalucci D, Sporeno E, Cirillo A, Ciliberto G, Nicosia A, Colloca S . An adenovirus type 5 (Ad5) amplicon-based packaging cell line for production of high-capacity helper-independent deltaE1-E2-E3-E4 Ad5 vectors. J Virol 2005; 79: 6400–6409.
Aste-Amezaga M, Bett AJ, Wang F, Casimiro DR, Antonello JM, Patel DK et al. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Hum Gene Ther 2004; 15: 293–304.
Zucchelli S, Capone S, Fattori E, Folgori A, Di Marco A, Casimiro D et al. Enhancing B- and T-cell immune response to a hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J Virol 2000; 24: 11598–11607.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fattori, E., Zampaglione, I., Arcuri, M. et al. Efficient immunization of rhesus macaques with an HCV candidate vaccine by heterologous priming–boosting with novel adenoviral vectors based on different serotypes. Gene Ther 13, 1088–1096 (2006). https://doi.org/10.1038/sj.gt.3302754
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302754
Keywords
This article is cited by
-
Genetic Vaccine for Respiratory Syncytial Virus Provides Protection Without Disease Potentiation
Molecular Therapy (2014)
-
The Novel Replication-defective Vaccinia Virus (Tiantan Strain)–based Hepatitis C Virus Vaccine Induces Robust Immunity in Macaques
Molecular Therapy (2013)
-
Viruses as vaccine vectors for infectious diseases and cancer
Nature Reviews Microbiology (2010)
-
New Insights on Adenovirus as Vaccine Vectors
Molecular Therapy (2009)