Abstract
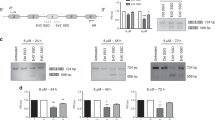
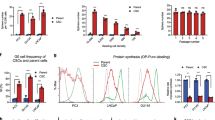
An important factor implicated in tumor cell predisposition for invasion and metastasis is the malignancy-related upregulation of urokinase plasminogen activator receptor (uPAR). uPAR signals by activating different tyrosine kinases in different cells. We examined the effects of inhibiting uPAR signaling by inhibition of uPAR expression with antisense oligonucleotides (aODNs) in PC3 human prostate cancer cells and evaluated aODN effect in a mouse model of prostate cancer bone metastasis. Following uPAR aODN treatment, PC3 cells exhibited a strong decrease in uPAR expression, evaluated by flow cytometry and by polymerase chain reaction, and of FAK/JNK/Jun phosphorylation. The synthesis of cyclins A, B, D1 and D3 was inhibited, as shown by Western blotting, flow cytometry and polymerase chain reaction, and PC3 cells accumulated in the G2 phase of the cell cycle. PC3 cells' adhesion was unaffected, while proliferation and invasion of Matrigel were impaired. A total of 60 mice were subjected to intracardiac injection of PC3 cells and were randomly assigned to three groups: aODN (treated with 0.5 mg intraperitoneum/mouse/day), dODN (treated with the same amounts of a degenerated ODN) and control (injected with a saline solution). At 28 days after heart injection, mice were subjected to a digital scan of total body radiography, which revealed 80% reduction in mice affected by bone metastasis. The use of uPAR aODNs produced a substantial prophylactic effect against prostate cancer bone metastasis, which has to be ascribed to downregulation of uPAR expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ellis WJ et al. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology 2003; 61: 277–281.
Andreasen PA, Egelund R, Petersen HH . The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci 2000; 57: 25–40.
Mignatti P, Rifkin DB . Nonenzymatic interactions between proteinases and the cell surface: novel roles in normal and malignant cell physiology. Adv Cancer Res 2000; 78: 103–157.
Del Rosso M et al. Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metast 2002; 19: 193–207.
Aguirre Ghiso JA, Kovalski K, Ossowski L . Tumor dormancy induced by down-regulation of urokinase receptor in human carcinoma involves integrin and MAPK signalling. J Cell Biol 1999; 147: 89–104.
Aguirre Ghiso JA . Inhibition of FAK signalling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene 2002; 21: 2513–2524.
Aguirre Ghiso JA et al. Urokinase receptor and fibronectin regulate the ERK (MAPK) to p38 (MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell 2001; 12: 863–879.
Quattrone A et al. Reversion of the invasive phenotype of transformed human fibroblasts by anti-messenger oligonucleotide inhibition of urokinase receptor gene expression. Cancer Res 1995; 55: 90–95.
Fibbi G et al. Functions of the fibrinolytic system in human Ito cells and its control by basic fibroblast and platelet-derived growth factor. Hepatology 1999; 29: 868–878.
Aguirre-Ghiso JA, Estrada Y, Lu D, Ossowski L . ERKMAPK activity as a determinant of tumor growth and dormancy; regulation by p38SAPK. Cancer Res 2003; 63: 1684–1695.
Goltzman D . Mechanisms of the development of osteoblastic metastases. Cancer 1997; 80 (Suppl. 8): 1581–1587.
Rabbani SA, Xing RH . Role of urokinase (uPA) and its receptor (uPAR) in invasion and metastasis of hormone-dependent malignancies. Int J Oncol 1998; 12: 911–920.
Festuccia C et al. Plasminogen activator system modulates invasive capacity and proliferation in prostatic tumor cells. Clin Exp Metast 1998; 16: 513–528.
Gavrilov D et al. Expression of urokinase plasminogen activator and receptor in conjunction with the ets family and AP-1 complex transcription factors in high grade prostate cancers. Eur J Cancer 2001; 37: 1033–1040.
Miyake H et al. Elevation of serum levels of urokinase-type plasminogen activator and its receptor is associated with disease progression and prognosis in patients with prostate cancer. Prostate 1999; 39: 123–129.
Magdolen V et al. Inhibition of the tumor-associated urokinase-type plasminogen activation system: effects of high-level synthesis of soluble urokinase receptor in ovarian and breast cancer cells in vitro and in vivo. Recent Results Cancer Res 2003; 162: 43–63.
Kobayashi H et al. Bikunin plus paclitaxel markedly reduces tumor burden and ascites in mouse model of ovarian cancer. Int J Cancer 2004; 110: 134–139.
D'Alessio S et al. Antisense oligodeoxynucleotides for urokinase-plasminogen activator receptor have anti-invasive and anti-proliferative effects in vitro and inhibit spontaneous metastases of human melanoma in mice. Int J Cancer 2004; 110: 125–133.
Yang YM et al. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res 2003; 9: 391–401.
Shaulian E, Karin M . AP-1 in cell proliferation and survival. Oncogene 2001; 20: 2390–2400.
Schwabe RF et al. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology 2003; 37: 824–832.
Bakiri L, Lallemand D, Bossy-Wetzel E, Yaniv M . Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J 2000; 19: 2056–2068.
Oktay M et al. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol 1999; 145: 1461–1469.
Bohuslav J et al. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med 1995; 181: 1381–1390.
Xue W, Kindzelskii AL, Todd III RF, Petty HR . Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol 1994; 152: 4630–4640.
Wei Y et al. Regulation of integrin function by the urokinase receptor. Science 1996; 273: 1551–1555.
Montuori N et al. The cleavage of the urokinase receptor regulates its multiple functions. J Biol Chem 2002; 277: 46932–46939.
Koshelnick Y et al. Urokinase receptor is associated with the components of the JAK1/STAT1 signaling pathway and leads to activation of this pathway upon receptor clustering in the human kidney epithelial tumor cell line TCL-598. J Biol Chem 1997; 272: 28563–28567.
Behrendt N et al. A urokinase receptor-associated protein with specific collagen binding properties. J Biol Chem 2000; 275: 1993–2002.
Ossowski L, Aguirre-Ghiso JA . Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr Opin Cell Biol 2000; 12: 613–620.
Blasi F, Carmeliet P . uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002; 3: 932–943.
Lucia MS et al. Workgroup I: rodent models of prostate cancer. Prostate 1998; 36: 49–55.
Soos G et al. Heterotypic growth of human prostate carcinoma in the femurs of nude mice: an osseous metastatic model. Int J Cancer 1996; 66: 280–281.
Shevrin DH, Kukreja SC, Ghosh L, Lad TE . Development of skeletal metastasis by human prostate cancer in athymic nude mice. Clin Exp Metast 1988; 6: 401–409.
Wu TT et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 1998; 77: 887–894.
Arguello F, Baggs RB, Frantz CN . A murine model of experimental metastasis to bone and bone marrow. Cancer Res 1988; 48: 6876–6881.
Wetterwald A et al. Optical imaging of cancer metastasis to bone marrow: a mouse model of minimal residual disease. Am J Pathol 2002; 160: 1143–1153.
Corey E et al. Establishment and characterization of osseous prostate cancer models: intra-tibial injection of human prostate cancer cells. Prostate 2002; 52: 20–33.
Shetty S, Kumar A, Johnson AR, Idell S . Regulation of mesothelial cell mitogenesis by antisense oligonucleotides for the urokinase receptor. Antisense Res Dev 1995; 5: 307–314.
Kook YH, Adamski J, Zelent A, Ossowski L . The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy. EMBO J 1994; 13: 3983–3991.
Go Y et al. Inhibition of in vivo tumorigenicity and invasiveness of a human glioblastoma cell line transfected with antisense uPAR vectors. Clin Exp Metasts 1997; 15: 440–446.
Fibbi G et al. Urokinase-dependent angiogenesis in vitro and diacylglycerol production are blocked by antisense oligonucleotides against the urokinase receptor. Lab Invest 1998; 78: 1109–1119.
Annunziato F et al. MDC and ELC attract human thymocytes in different stages of development and are produced by distinct subsets of medullary epithelial cells: possible implications for negative selection. J Immunol 2000; 165: 238–246.
Romagnani P et al. Interferon-inducible protein 10, monokine induced by interferon γ, and interpheron-inducible T-cell α chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) αβ1 CD8+ single-positive T cells, TCRγδ1 T cells, and natural killer-type cells in human thymus. Blood 2001; 97: 601–607.
Romagnani P et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest 2001; 107: 23–33.
Arguello F, Baggs RB, Frantz CN . A murine model of experimental metastasis to bone and bone marrow. Cancer Res 1988; 48: 6876–6881.
Agrawal S, Temsamani J, Tang JY . Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci USA 1991; 88: 7595–7599.
Acknowledgements
This study was supported by grants from University of Florence, and Ente Cassa di Risparmio of Florence. We acknowledge the skilful technical assistance of Mr Marco Cutri of the Department of Experimental Pathology and Oncology (University of Florence) with computer programs.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Margheri, F., D'Alessio, S., Serratí, S. et al. Effects of blocking urokinase receptor signaling by antisense oligonucleotides in a mouse model of experimental prostate cancer bone metastases. Gene Ther 12, 702–714 (2005). https://doi.org/10.1038/sj.gt.3302456
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302456
Keywords
This article is cited by
-
uPAR antibody (huATN-658) and Zometa reduce breast cancer growth and skeletal lesions
Bone Research (2020)
-
Extracellular Vesicles: How to Shuttle the Metastatic Programme
Current Molecular Biology Reports (2019)
-
Translational models of prostate cancer bone metastasis
Nature Reviews Urology (2018)
-
Spontaneous lung and lymph node metastasis in transgenic breast cancer is independent of the urokinase receptor uPAR
Clinical & Experimental Metastasis (2015)
-
Urokinase-type plasminogen activator receptor regulates apoptotic sensitivity of colon cancer HCT116 cell line to TRAIL via JNK-p53 pathway
Apoptosis (2014)