Key Points
- Five perendoscopic antireflux devices have received Food and Drug Administration (FDA) approval for clinical use since 2000. Two have been subsequently withdrawn from the market by the manufacturers owing to safety and other concerns.
- Antireflux defenses at the gastroesophageal junction are the targets of endoscopic therapeutic techniques.
- Perendoscopic anti–gastroesophageal reflux disease (GERD) techniques are described in detail.
- The great majority of publications on clinical trials of antireflux devices have been abstracts, anecdotal experiences, or open-labeled, uncontrolled studies. Results of only two randomized studies have been reported.
- The GERD patients involved in antireflux endoscopic treatment trials are a highly selective patient population.
- Endoscopic techniques to treat GERD improve symptoms often without reducing or normalizing acid reflux.
- Promising features and problems of endo-GERD treatments are outlined. Safety and efficacy issues persist as evident by the manufacturers' withdrawal of two FDA-approved devices.
- Criteria for premarketing of future endoscopic GERD devices are recommended.
Introduction
Perendoscopic therapy has assumed a role in the treatment paradigm for gastroesophageal reflux disease (GERD) patients. A variety of technical procedures delivered through or with the assistance of an endoscope to prevent gastroesophageal reflux have been introduced since the beginning of the 21st century (Table 1). Five devices have received Federal Drug Administration (FDA) approval for clinical use within the last 5 years. Positive reports of therapeutic outcomes have been numerous and worldwide1; thousands of patient have been treated for their GERD disorder by endoscopic techniques. However, issues of premature marketing and treatment efficacy and safety involved with these procedures have raised concerns,2, 3, 4, 5 and two of the FDA-approved devices have been withdrawn from the market by the manufacturers. This chapter reviews these unique endoscopic devices and procedures and discusses their clinical promise and problems.
Esophageal Gastroesophageal Reflux Barriers
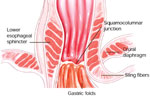
The physiologic and structural antireflux defenses at the gastroesophageal junction are the targets of endoscopic therapeutic techniques6 (Figure 1).
The distal esophagus is protected by a sphincteric apparatus consisting of two components: a smooth muscle (intrinsic) lower esophageal sphincter (LES) that is the primary barrier to gastroesophageal reflux (GER) and a skeletal (extrinsic) diaphragmatic crus. The LES zone is approximately 4.0 cm in length. The proximal portion begins 1 to 2 cm above the squamocolumnar interphase and is enveloped by the crural diaphragm; the distal LES resides within the abdominal cavity. The LES maintains a constant tone owing to its intrinsic muscular properties reinforced by excitatory cholinergic activity through the vagus nerve.
Gastric contents can reflux into the esophagus when the LES pressure is hypotensive or during transient LES relaxations (TLESRs),7 which are caused by a vagovagal reflex triggered by gastric distention. The afferent sensory field is located in the gastric cardia and fundus. Specialized terminal endings of vagal fibers that are "deformity sensitive" appear to be responsible for both LES receptive relaxation and TLESR initiation.8
Sling or oblique fibers located beneath the LES zone in the gastric fundus reinforce the antireflux barriers. These sling fibers are in a "C" configuration resulting in a "flap-valve" design. This flap valve mechanism reinforces the LES tone when gastric fundic pressure is increased.
Severe esophagitis is frequently associated with a hiatal hernia. A hiatal hernia may vary in size; it may be large and fixed within the chest or small enough to slide to and fro into the abdomen. Its presence may enhance gastric reflux by impairing the sphincteric function of the diaphragmatic crus, compartmentalizing acid in proximity to the distal esophagus and negating the flap valve mechanism and intraabdominal location of the LES.9
Perendoscopic anti–gastroesophageal reflux disease techniques
Perendoscopic GERD treatments are designed to augment the natural reflux barriers already described. Three basic techniques are in use: (1) radiofrequency (RF) thermal energy; (2) sewing or plication; and (3) implantable or injectable biopolymers. Adaptation of these unique technologies to anti-GERD procedures is reviewed in detail for subsequent appreciation of clinical efficacy and safety.
Radiofrequency Thermal Energy Application
Radiofrequency ablation procedures have been used in the past to treat a number of clinical problems ranging from tumor ablation to Wolff-Parkinson-White syndrome.10 Animal studies suggest RF energy applied to the canine distal esophagus resulted in hypertrophy and fibrosis of adjacent muscle and thickening of the gastric cardia. Human investigations have demonstrated reduction of TLESRs, sensory acuity, and compliance of the LES zone.11
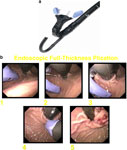
The Stretta system (Curon Medical Inc., Sunnyvale, CA) consists of an RF catheter delivery system with a distal inflation balloon covered by four thin electrode sheaths arranged radically at 90-degree increments (Figure 2). A 5.5-mm curved needle electrode is contained within each sheath and is deployed within the distal esophageal tissue during balloon inflation. A four-channel control module regulates RF energy output while constantly monitoring tissue temperature from thermocouples at the base and tip of the needle. The esophageal squamocolumnar junction (SGJ) location is identified at endoscopy where a guidewire is placed. The Stretta catheter is inserted over the wire and positioned with the proximal pole of the balloon 1.0 cm above the SGJ based on graduated tube markings. During subsequent balloon inflation and electrode deployment, RF energy is applied for 90 seconds. Following completion of the first set of RF lesions, the balloon is deflated, the needles retracted, and the catheter rotated 45 degrees. A second set of lesions is made, resulting in a circular ring of eight RF thermal burns. A total of 14 sets of RF lesions (56 sites) are made from 0.5 cm above the SGJ to the gastric cardia, delivering a total of 21 minutes of RF thermal energy. Estimated procedure time is approximately 1 hour.
Figure 2: The Stretta radiofrequency delivery system.
a: Lower esophageal sphincter (LES) zone pretreatment. b: Catheter insertion. c: Initial axial burn. d: Second axial burn; 45°C burn. e: Completion of RF treatments (eight per axial level) above/below gastroesophageal junction (GEJ). f: LES zone posttreatment. (Source: Curon Medical, Inc. Fremont, CA, with permission)
Endoscopic Suturing (Plication)
Submucosal suturing The EndoCinch suturing system (CR Bard, Inc., Murray Hill, NJ) is designed to alter gastroesophageal junction (GEJ) anatomy by suturing together gastric cardia folds along the lesser curvature accentuating the angle of HIS. The original system contained a miniature sewing capsule that was attached to the endoscope, a knot pusher, metal-tagged sutures, and cutter. The need for tying knots, cutting suture ends, and cinching together the tagged parts at the surface of the gastric tissue have been obviated with introduction of a small ring and peg-cinching device on the current devices.
Following the insertion of an overtube over a 15-mm dilator, an endoscope with a distally mounted sewing capsule is passed below the GEJ to a desired depth. Gastric tissue is aspirated into the sewing capsule, and a hollow-core needle with a metal tilt-tag attached to a suture is then advanced through the suctioned tissue. A stiff wire is pushed through the hollow needle by the control handle attached to the biopsy port, driving the metal tilt-tag forward to be captured into the tip of the sewing capsule. The system is withdrawn through the overtube together with the tilt-tag attached to the suture. The same tilt-tag suture is reloaded into the hollow core needle and the suture procedure is repeated. The second stitch is placed 1 to 1.5 cm away from the initial stitch and the two are approximated and cinched by the ceramic plug and ring attached to the second endoscope. Two to three plications are placed during the EndoCinch procedure. The stitches are usually placed in the submucosa 1 cm below the GEJ in a circular array at the 3, 6, and 9 o'clock positions. The EndoCinch procedure takes an average of 45 minutes (range 25 to 100 minutes).
Full-thickness suturing The full-thickness endoscopic suturing system (Plicator NDO Surgical Inc., Mansfield, MA) is a two-channel instrument with a control handle that provides retroflexion of the tube, operates opening and closing of the distal jaw-like arms, and deploys a suture implant (Figure 3). A stainless steel helical retractor penetrates a gastric fold to the depth of the serosa and pulls the gastric wall into the opened jaws. The procedure is viewed by a 5.9-mm pediatric endoscope inserted through the second channel. The suture implant affixed to the jaws consists of suture prethreaded onto two titanium retention bridges. The suture bolsters are made of soft, expandable fluoroethylene materials. A video prepared by the vendor can be viewed at http://www.ndosurgical.com/.
Figure 3: The Plicator system.
a: Plicator instrument. b: Plicator: full-thickness plication sequence. (Source: NDO Surgical, Inc., with permission)
The Plicator is passed through a 60-French overtube to a level 10 to 12 cm below the GEJ. The corkscrew retractor penetrates the stomach wall 1 to 2 cm below the GEJ. The jaws are closed (est. 5-cm bite), forming one large plication consisting of two full-thickness segments of gastric cardia. The mean procedure time is approximately 21 minutes.
Injection/Implantation
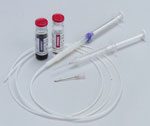
Injection Enteryx injection therapy (Boston Scientific, Natich, MA) utilizes a biocompatible polymer mixed with radiopaque contrast agent (tantalum powder) dissolved in an organic liquid carrier (dimethylsulfoxide, DMSO). Upon injection and contact with tissue, the DMSO diffuses into tissue and precipitates the polymer as a spongy mass.(Figure 4).
Figure 4: Enteryx: polymer injectable materials.
(Source: Minnesota Gastroenterology, P.A., St. Paul, MN, with permission)
Enteryx injection is performed under fluoroscopic guidance. The injection catheter, introduced through the biopsy channel of an endoscope, penetrates into the muscle or deep submucosal layer of the cardia at or 1 to 2 mm below the SCJ. If the polymer spreads into an arc (confirmed by fluoroscopy), additional material is injected at the same site. Otherwise, multiple 1- to 2-cc injections are performed in circumferential fashion. A total of 6 to 8 cc of Enteryx is injected at the rate limited to 1 cc/min to allow polymerization and dissipation of heat. If tissue depth of injection is shallow, a new site of injection is required. The average procedure time is 33 minutes, (Figure 5).
Implantation The Gatekeeper (Medtronics, Minneapolis, MN) technique places miniature expandable hydrogel prosthesis at the GEJ. The device comprises a 16-mm overtube with a distal rigid segment containing a shelf-like structure through which an endoscope is inserted. The system is passed to the GEJ. Suction applied through the endoscope retracts tissue into the bottom shelf of the overtube A needle catheter inserted through a second channel in the overtube creates a submucosal bleb with saline injection. Subsequently, a prosthesis delivery tube is passed through the channel to the bleb site where a slit has been formed. The sheath portion of the delivery system remains within the submucosal slit following removal of the needle/dilator system. A desiccated 1.5  18 mm hydrogel prosthesis is inserted into the proximal opening of the sheath and advanced by a push rod to the submucosal slit. Although 12 prostheses have been placed at one sitting, the average number of hydrogel pledgets placed is six. The procedure averages 15 minutes for initial prosthesis placement and 5 minutes for each additional placement.
18 mm hydrogel prosthesis is inserted into the proximal opening of the sheath and advanced by a push rod to the submucosal slit. Although 12 prostheses have been placed at one sitting, the average number of hydrogel pledgets placed is six. The procedure averages 15 minutes for initial prosthesis placement and 5 minutes for each additional placement.
Overview of Clinical Trials
A myriad of reports have been published about the results of endoscopic treatment trials of GERD. The great majority of the publications have been abstracts, anecdotal experiences, or open-labeled uncontrolled studies frequently recounting positive patient outcomes following endoscopic GERD therapy.
Patients enrolled in these clinical trials have uniformly had symptoms of heartburn and regurgitation documented by validated questionnaires, for example, GERD–Health Related Quality of Life (GERD-HRQL) scores and qualify of life issues obtained by the medical outcomes study using the Short Form 36 (SF-36). Patients have been taking daily antacid medication with symptomatic responses ranging from partial to complete. Esophageal acid exposure of >5% duration on 24-hour ambulatory pH recording is required for many of these endoscopic GERD therapy trials.12, 13, 14
Clinical trials evaluating endoscopic GERD therapy thus far have included a highly selective patient population. Patients are excluded if they have a hiatal hernia >2.0 cm or esophageal inflammation greater than grade II on the Los Angeles Esophagitis Classification. Subsequent endoscopic assessment of the esophageal lining following endoscopic GERD treatment is not mandatory. The primary goal of these clinical trials is reduction in proton pump inhibitor (PPI) usage. Secondary outcomes are improvement in GERD-HRQL symptom scores and esophageal acid exposure.
The apparent symptom improvement in many patients enrolled in open-label trials following endoscopic GERD treatments has been subject to criticism.4 One authority on GERD, Dr. Peter Kahrilas,15 wrote, "With a disease (GERD) that can have a 60% placebo response rate, it is simply not good enough to equate a 60% symptomatic response in an uncontrolled trial." Schoenfield and Sherman16 are very explicit in their concerns regarding the composition of an appropriately designed treatment trial: "Randomization, concealed allocation, and double-blinding are the only study design techniques proven to prevent an inflated estimate about the benefits of treatment." In a recent editorial concerning endoscopic antireflux procedures, Dr. Nicholas Shaheen4 states: "Early publications dwell more on methodological aspects of the performance of the procedure than on justifying the outcomes measured on the analysis performed." For these reasons, we begin this review citing the two most meaningful published results to date on the outcomes of endoscopic GERD treatments obtained in a randomized blinded clinical trial.
Randomized Trials
The initial randomized sham controlled trial involved the Stretta RF procedure 3 years after its introduction into the marketplace.17 The study enrolled 64 patients at eight participating centers. The Stretta RF treatment was applied to the esophagus of 35 patients, and a sham procedure was used on 29 patients. The RF treatment group demonstrated significant improvement in heartburn symptoms and quality of life scores at 6- and 12-month follow-up. At 6 months, patients in the sham group were permitted to cross over to RF therapy. At that point, however, no significant difference was demonstrated in reduction of daily antacid medication or total esophageal acid exposure between the two groups.
The results of a second randomized sham controlled multicenter study evaluating an endoscopic treatment technique for GERD have been published recently.18 The Enteryx technique was assessed in 32 patients, and another group of 32 patients received a sham application. Prior to study participation, the patient's GERD symptoms were "well controlled by PPI therapy and rapidly recurred after cessation of PPI therapy." The primary study goal was a  50% reduction in PPI usage. A secondary goal was
50% reduction in PPI usage. A secondary goal was  50% improvement in GERD score and frequency of retreatment (patients in both groups with continuing symptoms after 3 months were eligible for Enteryx retreatment).
50% improvement in GERD score and frequency of retreatment (patients in both groups with continuing symptoms after 3 months were eligible for Enteryx retreatment).
Twenty-five (78%) Enteryx treated patients achieved  50% reduction in PPI use compared to 17 patients (53%) in the sham group. Twenty-one patients (65%) in the Enteryx group stopped PPI use completely compared to 13 patients (41%) in the sham group. The GERD-HRQL heartburn scores also improved significantly in the Enteryx group (67% versus 22%). Retreatment was significantly less in the Enteryx group. Decreased PPI use and symptomatology remained stable at 6 months in the Enteryx group. However, similar to the Stretta study, no significant changes in esophageal acid exposure was noted when baseline studies were compared to 6-month studies in the Enteryx treatment group.
50% reduction in PPI use compared to 17 patients (53%) in the sham group. Twenty-one patients (65%) in the Enteryx group stopped PPI use completely compared to 13 patients (41%) in the sham group. The GERD-HRQL heartburn scores also improved significantly in the Enteryx group (67% versus 22%). Retreatment was significantly less in the Enteryx group. Decreased PPI use and symptomatology remained stable at 6 months in the Enteryx group. However, similar to the Stretta study, no significant changes in esophageal acid exposure was noted when baseline studies were compared to 6-month studies in the Enteryx treatment group.
Open-Labeled Trials
An exhaustive review of publications since 2001 on the outcomes of open-labeled studies using endoscopic GERD devices has been published recently.1 This summary includes the results of sewing devices, RF ablation, and injection treatments on GERD patients. There are roughly 18 published papers and 48 abstracts. The authors conclude that "convincing" results have been obtained in only two thirds of patients with a median follow-up of 6 months. They point out the number of inconsistencies between treatment efficacy in relationship to improvement of symptoms and quality of life and the absence of improvement in objective parameters such as LES pressure and esophageal acid exposure. Nonetheless, there were five endoluminal therapies that have received regulatory approval (FDA) in the U.S. (Stretta, EndoCinch, Enteryx, NDO Plicator, and the Gatekeeper System).19 Peer-reviewed publications on the efficacy and safety of these endoscopic techniques have been forthcoming and these studies need to be addressed despite the obvious flaws in protocol design.
EndoCinch
Endoluminal gastroplication (EndoCinch) therapy was evaluated in a 24-month prospective, open-label multicenter trial.20 Eighty-five patients were enrolled at five centers in the U.S. The patients were dependent on histamine-2 receptor antagonists and/or PPIs for symptom control. At 1 and 2 years, patients had significant reduction in heartburn scores and regurgitation symptoms. At 12 and 24 months, heartburn symptoms resolved in 59% and 52% of patients, respectively, whereas regurgitation was eliminated in 83% and 77% of patients at similar periods. Elimination or reduction of PPI use was achieved at 12 months in 73% of patients and 69% at 24 months.
The median annual per-patient cost for PPI medication was estimated by the investigators to be $1564. Twelve months following the procedure, the estimated annualized median cost was $157 and remained at this approximate level at 24 months, for an estimated cost reduction of 90%. Twenty-seven of the 68 patients (39.7%) normalized esophageal pH values at both 3 and 6 months following EndoCinch treatment. The authors point out that the 24-month results indicate a trend toward increased symptoms compared to those at 12 months. No mention is made about the durability of the implanted sutures or the frequency of repeat procedures.
Enteryx
The results of the open-label multicenter international clinical trial evaluating Enteryx implantation therapy for PPI-dependent GERD patients has been updated and reported recently.14 Currently 144 patients have been followed at 6- and 12-month intervals after the Enteryx procedure. Sixty-four patients evaluated at 24 months are also enrolled in the 36-month post Enteryx approval study mandated by the FDA for U.S. study participants.
Patients entered the trial if their symptoms normalized, were well-controlled on PPI therapy, and regressed to abnormal levels within 10 days of eliminating antacid use. Treatment responders met the primary outcome goal if PPI use was eliminated or reduced  50% after Enteryx implantation. Twenty-one patients (15%) were permitted to enter the trial who did not fulfill one or more of selection criteria; for example, they had a hiatal hernia
50% after Enteryx implantation. Twenty-one patients (15%) were permitted to enter the trial who did not fulfill one or more of selection criteria; for example, they had a hiatal hernia  3 cm in length (12 patients) or absence of symptom recurrence off of PPI (five patients).
3 cm in length (12 patients) or absence of symptom recurrence off of PPI (five patients).
Treatment responders with  50% reduction in PPI usage constituted 84% of the trial population at 12 months per protocol and 78% by intent-to-treat analysis. Cessation of PPI use was achieved in 73% and 68% of patients, respectively. Exclusion of the 21 patients with protocol deviations had negligible impact on the study results. The treatment response rate at 24 months in the 64 postapproval study patients was 72%, and cessation of PPI use was 67%. Eighteen of these 64 patients were judged nonresponders.
50% reduction in PPI usage constituted 84% of the trial population at 12 months per protocol and 78% by intent-to-treat analysis. Cessation of PPI use was achieved in 73% and 68% of patients, respectively. Exclusion of the 21 patients with protocol deviations had negligible impact on the study results. The treatment response rate at 24 months in the 64 postapproval study patients was 72%, and cessation of PPI use was 67%. Eighteen of these 64 patients were judged nonresponders.
The GERD-HRQL symptoms were improved in 78% of the 144 patient groups at 12 months; the median regurgitation improvement score was 77%. The median supine, upright, and total times that the pH was less than 4.0 decreased by 42%, 28%, and 31%, respectively at 12 months. Normalization of esophageal pH occurred in 37% following Enteryx therapy. Interestingly, an increase in the severity of esophagitis was observed in 34 of 107 patients (32%). Of 96 patients with a baseline esophagitis score of 0 to 1 (Savary classification) at endoscopy, 26% progressed to grade II at 12 months following endotherapy. The residual Enteryx polymer implant volumes at 12 months was estimated by unblinded investigators to be 67% (mean volume of injected implanted Enteryx was 6.9 mL). Thirty-seven patients (26%) required repeat implantation between 1 to 3 months after the original implantation procedure.
In this study, the authors note, "At least one adverse event occurred in each of the 144 patients." The most common morbidity was chest pain (85% of patients), which generally resolved in 2 weeks. Dysphagia was experienced by 24% of patients.
Plicator
The 12-month results of endoscopic full-thickness plication treatment have been reported recently in an open-label North American trial.21 A total of 64 patients received a transmural plication at the time of enrollment. Fifty-seven patients completed the 12-month follow-up. Ambulatory 24-hour pH monitoring and esophageal manometry were obtained at entry and at 3-month follow-up. The pH study was repeated at 6 months. Treatment goals were similar to those of other open-label trials.
Forty patients (70%) were no longer taking a PPI medication at 12 months follow-up. The GERD-HRQL scores were improved also. At the 6-month follow-up, distal esophageal acid exposure improved in 40 of 51 (78%), with a 39% decrease in the mean percentage of time the pH was <4.0. Normalized pH scores were observed in 30% of patients.
Gatekeeper
Preliminary results of the Gatekeeper full-thickness plicator in GERD patients were initially published in 2003.22 In a pilot study of seven patients, the primary goals of the study were procedure safety, feasibility, and long-term durability of full-thickness tissue fixation associated with the procedure. At 6 months, the plication was intact in all patients. The following year the results of open-label multicenter study involving 68 GERD patients treated with the Gatekeeper reflux repair system were published.23 Forty-nine of the patients were followed for 6 months. Fifty-three percent of patients were off PPI medication at that point, although only 70% of the prostheses were intact. There was improvement in esophageal acid exposure and regurgitation. The most recent publication of the Gatekeeper therapy involved nine GERD patients with a far different goal: the spatiotemporal features of gastroesophageal refluxate following esophageal placement of the hydrogel prosthesis.24 Twenty-four-hour pH studies were performed on the study group before and 6 months after Gatekeeper treatment. The dynamic characteristics of acid reflux in the patients group was compared to those of 13 control subjects without symptoms. In patients, acid exposure time in the distal esophagus decreased, but not significantly. However, the proximal extent of acid reflux in the patient group decreased from 37.5% at baseline to 9.5% at 6 months following endotherapy. (Proximal extent in the healthy control group was 9.8%.) The results appear to support prior studies, which have shown that the spread of gastric acid within the proximal esophagus is a significant factor triggering symptoms in GERD patients.25 The authors believe this positive effect of Gatekeeper therapy may explain the improvement in symptom treated by this technique.
Endo–Gastroesophageal Reflux Disease Treatments: The Promise
Endoscopic techniques to treat GERD patients have for the most part resulted in improving symptoms, often without reducing or normalizing acid reflux.
The Stretta procedure has resulted in reduction in frequency of TLESRs,11 probably causing neurolysis26 or remodeling (stiffening) tissue at the gastroesophageal junction. Subsequently, the volume of acid refluxate may be diminished, and loss of sensation to low pH exposure may account for the majority of improvement related to this procedure.
Explanations of the therapeutic results of a recent Gatekeeper study are based on the findings of a significant decrease in acid reflux at the proximal and mid-esophagus, not the distal esophagus. In addition, the longer duration of proximal reflux events in the supine position, reported to be a pathophysiologic marker in GERD patients,27 was decreased after implantation therapy. These findings provide a mechanistic answer for the apparent discrepancy in symptom improvement in the face of unchanged distal acid exposure.
Another feature that may explain the therapeutic effectiveness of implantation therapy in the distal esophagus is the remodeling of the esophagogastric junction opening anomalies recently described in GERD patients compared to normals.28 This would negatively impact the accessibility and the volume of gastric reflux entering the esophagus.
The LES pressure is the most important deterrent to gastroesophageal reflux.6 A logical explanation for reduction in acid exposure and improved symptoms following endo-GERD therapy would be an increase in LES pressure. However, neither Stretta nor EndoCinch procedures caused significant changes in LES pressure,29, 30 whereas Enteryx therapy resulted in a mild increase in LES tone following relaxation.31 Both Enteryx and EndoCinch studies reported an increase in the length of the LES zone after therapy, but this effect on reflux is questionable.
Comparison of the three endotherapies (Enteryx, Plicator and EndoCinch)14, 20, 21 demonstrated effectiveness in decreasing subsequent PPI usage in all three studies in follow-up periods ranging from 12 to 24 months. However, only one of the three studies (Enteryx) included any information about esophageal inflammatory changes. Esophagitis grading remained unchanged in 55% of patient, but increased in 32% after the Enteryx procedure. Finally, retreatment was not mentioned in these three back-to-back publications, but may be required in patients undergoing Enteryx and gastroplication. Retreatment is not an option in patients receiving Plicator treatment.
Endoscopic treatments have been used on a restrictive minority of GERD patients to date. Entrance criteria for patients involved in the vast majority of endo-GERD treatment trials have been uniformly limited to a small hiatal hernia and virtually no erosive esophagitis. In fact, a large group of 474 GERD patients were evaluated retrospectively to determine potential eligibility for endoscopic treatment using the standard selection criteria for patient enrollment in these trials.32 Only 88 patients (19%) fulfilled these eligibility criteria. Unless these endo-GERD treatments become applicable to a more heterogeneous GERD population including those with a larger hiatal hernia and severe erosive esophagitis, their role in the GERD treatment paradigm will be marginalized.
Potential Candidates for Endo–Gastroesophageal Reflux Disease Therapy
Candidates suited for GERD endotherapy are best reflected in the following categories33: patients with quality of life impairment owing to heartburn who require escalating PPI doses; patients on PPIs with residual regurgitation; patients who cannot tolerate PPI medications; patients fearful of long-term sequela from PPIs; and postfundoplication patients with an intact wrap who have recurrent symptoms. Validation of these arbitrary selection criteria await appropriately designed and controlled outcome studies.
Endo–Gastroesophageal Reflux Disease Treatments: The Problem
Safety and Technical Issues
Endotherapy for GERD has been associated with significant morbidity and mortality in the clinical trials to date. Voluntary reporting of possible complications following endotherapy techniques can be registered on the FDA-sponsored Web site via the Manufacturers and Users Facility Device Experience Database (MAUDE). Impressive complications of endo-GERD treatments have been reported subsequently; for example, the Stretta procedure has been associated with 23 significant complications including three deaths. Over 6000 patients have received Stretta treatment. The Enteryx procedure has been performed on approximately 2600 patients. It has been associated with 33 potential complications including five deaths; one death was attributed to an aortoesophageal fistula.4 Two complications reported recently after Enteryx injection include one patient who developed hemorrhagic pericarditis. Intense inflammation was noted along the medical border of the esophagus extending to the entire visceral pericardium at exploratory thoracotomy. Another patient developed a left pleural effusion and small right-pleural effusion, which resolved on medical therapy, but continued with significant dysphagia.34 Enteryx material was detected in the aorta and renal arteries of one patient after complaints of flank pain. Kidney function was not impaired apparently.18 Significant chest pain following Enteryx injection is a common sequela reported in all studies to date.14
On September 27, 2005, Boston Scientific Inc. withdrew the Enteryx system from the market because of safety concerns.
In a 12-month European EndoCinch study, "minor" adverse effects of sore throat, vomiting, abdominal pain, chest soreness, dysphagia, and bloating were described as "transient" and resolved spontaneously within 72 hours. Postprocedural bleeding occurred in two patients; one required transfusion.35 In the 1- to 2-year multicenter U.S. study of 85 patients, only seven patients experienced adverse events ranging from oozing at the suture site to postprocedure dysphagia.20
In a Gatekeeper trial, a pharyngeal perforation occurred in one patient owing to the overtube, whereas a second patient developed chronic nausea requiring the endoscopic removal of the prosthesis 3 weeks after implantation.23 Nonetheless, the Gatekeeper system has been withdrawn from the U.S. market because of lack of efficacy.
In an editorial in 2005, concerning the clinical use of endo-GERD techniques for treating acid reflux and the associated complications, the following question was posed: "What is the acceptable number of deaths and morbidity when treating proton-pump inhibitor responsive reflux with these devices?"4 The question has yet to be answered.
There remain a number of bothersome issues related to the use of endoscopic techniques to treat GERD patients. The distal esophagus is a vigorous, dynamic structure that shortens 1.5 cm with respirations and swallows; it may also be acutely angulated. These physiologic and structural features may present significant obstacles to accurate injection/implantation placement such as noted in the Enteryx complications. The distal esophageal wall is relatively thin and may not contain the 5-mm-length Stretta stylettes when they are deployed, allowing RF current to pass into adjacent structures.
Endo-GERD techniques pose unique problems. The Stretta relies on blind deployment of the RF needles. The Plicator and EndoCinch require a very large overtube, which is notorious for damage to the pliable esophagus, let alone one that is inflamed or strictured. Polymer injection (Enteryx) requires the use of radiology, and concerns about radiation exposure arise especially when repeated injections are required. Increased amounts of conscious sedation are required for these procedures; some endoscopists have resorted to the use of general anesthesia for appropriate patient compliance.
Finally, the very important issue of adequate training and experience to obtain competence in these procedures has been virtually ignored in the rush to market these instruments.36
Criteria for Premarketing of Future Endoscopic Gastroesophageal Reflux Disease Devices
Prior to approval of endo-GERD devices/procedures, there should be stringent FDA assessment of the device and procedure.37 This has not been the case in the past. Deaths from both Stretta and Enteryx procedures occurred in short-term studies of small patient groups shortly after marketing. More patient numbers and longer term follow-up should be mandated along with sham, controlled studies before approval of new endo-GERD technologies to ensure the efficacy and safety of the device. Both medical and surgical treatments of GERD patients have been proven effective in the past. A head-to-head comparison of these treatments would be useful and perhaps the best method of determining the role of endo-GERD procedures in the future treatment paradigm of GERD patients.
Based on several publications by authorities in the specialty, the current consensus on the present role of endoscopic treatment of GERD is best summed up by Dr. Jan Tack3: "For the time being endoscopic anti-reflux procedures should be done in a controlled environment, preferably in reference centers with adequately trained and experienced staff and within the framework of a registry or study."
Conclusion
Perendoscopic antireflux devices have been used to treat GERD patients for 5 years. Thousands of patients around the world have been treated for their GERD by endoscopic or endoscopic-assisted techniques. These devices and procedures have been described. The majority of clinical trials are open label, and positive results in symptom control and decreased use of PPI therapy were reported. However, objective parameters such as acid reflux normalization and elimination of esophagitis after endo-GERD treatment are infrequent findings. Only two randomized, controlled studies have been reported to date, and their findings were described in detail. Five perendoscopic anti-GERD procedures have received FDA approval; only three of these procedures remain available to clinicians at this time.
The potential and the problems associated with endoscopic antireflux therapies were discussed. The consensus of many authorities is that these remaining procedures are "not yet ready for prime time" in the clinical practice of gastrointestinal medicine.