Key Points
- Although gastroesophageal reflux disease (GERD) is a disorder of esophageal motility, the therapy of reflux disease is aimed at reducing gastric acidity in order to decrease the injurious effect of the refluxate to the esophageal mucosa.
- Lifestyle modifications may be effective in reducing or eliminating GERD symptoms, but the majority of patients require pharmacologic therapy. This varies with the severity of symptoms, and ranges from intermittent antacid therapy for mild disease to histamine-2 receptor antagonist (H2RA) therapy for moderate symptoms to daily protein pump inhibitor (PPI) therapy for severe symptomatic GERD. Continuous maintenance therapy with PPIs is the standard of care for severe GERD, but some patients may respond to intermittent short courses of PPI therapy given "on demand" when symptoms recur.
- Patients who are "refractory" to PPI therapy should have pH monitoring performed to confirm acid suppression. Causes of refractory symptoms include "functional" heartburn, bile acid reflux, and a hypersensitive esophageal mucosa that registers symptoms at low levels of acid exposure.
Introduction
Gastroesophageal reflux disease (GERD) affects more than 60 million Americans and remains one of the most common gastrointestinal disorders.1 Most affected individuals experience symptoms of GERD infrequently (less than once a month), but approximately 14% of Americans report GERD symptoms at least once per week, and up to 7% describe daily heartburn or regurgitation. Moreover, the extraesophageal manifestations of GERD, including asthma, chronic cough, and laryngitis, are being recognized with increasing frequency. Over the past 30 years, the management of GERD has evolved to the point at which the use of proton pump inhibitors (PPIs) provides highly effective treatment in even the most severe disease. Effective surgical therapies are available, and several new endoscopic therapies are being actively investigated; the mainstay of GERD treatment, however, remains the use of antisecretory medications, whose effectiveness and safety are well established.
Pathogenesis of Gastroesophageal Reflux Disease
Gastroesophageal reflux disease results from continued exposure of the esophageal mucosa to gastric secretions, particularly acid and pepsin. A number of anatomic and physiologic mechanisms normally prevent reflux from occurring, and derangement in any of these can promote esophageal acid exposure. The most important factors at work in preventing reflux include the lower esophageal sphincter (LES), esophageal clearance mechanisms that limit contact time with noxious substances, and mucosal protective factors intrinsic to the esophageal mucosa.
The LES, a 3- to 4-cm-long region of smooth muscle located at the esophagogastric junction, creates a zone of high pressure separating the esophageal and gastric compartments between swallows. The diaphragmatic crura assist the LES in the maintenance of a tonically closed sphincter. The contribution of hiatus hernia to GERD is well established; the hiatus hernia eliminates the contribution of the crural diaphragm to LES function and thereby promotes gastroesophageal reflux. The severity of reflux disease in patients with hiatal hernia has been positively correlated with the size of the hernia sac.2
The most common cause of gastroesophageal reflux is an excessive exposure of the esophagus to gastric secretions during transient lower esophageal sphincter relaxation (TLESR). These relaxations normally last for approximately 10 to 30 seconds, and occur in response to gastric distention and vagal stimulation. The purpose of TLESRs may be to vent gas from the stomach, and a TLESR is not necessarily associated with gastroesophageal reflux. Patients with GERD, however, have a higher frequency of TLESRs associated with reflux, and, as a consequence, a significantly prolonged duration of esophageal acid exposure.3 The contribution of TLESRs to GERD is greatest for patients with upright (daytime) reflux and nonerosive or mild erosive disease. Patients with more severe grades of esophagitis typically have other factors, such as hiatus hernia or decreased LES pressure, responsible for prolonged acid exposure.4
The entry of acid into the esophagus activates clearance mechanisms that limit exposure of the esophageal mucosa to gastric secretions. These include esophageal contractions (secondary peristalsis) that rapidly propel the refluxate into the stomach. Swallowing bicarbonate-rich saliva and secretion of bicarbonate by esophageal glands neutralizes any residual esophageal acid trapped in contact with the mucosa. Patients with either defective esophageal motility or decreased salivary secretion are predisposed to GERD.
Principles of Gastroesophageal Reflux Disease Therapy
Gastroesophageal reflux disease is primarily a disorder of esophageal motility, as most patients with GERD do not secrete abnormal quantities of gastric acid. The treatment of GERD, however, is typically not directed at the underlying pathophysiology but rather at reducing the acid content of the refluxate. If a less acidic fluid contacts the esophageal mucosa, the balance between offensive and defensive forces is shifted toward the side of mucosal protection. An effective therapy for GERD should accomplish three goals. The focus of treatment is control of symptoms, and most patients have complete symptom relief with appropriate medical therapy. An effective therapy should also heal esophageal mucosal damage (erosive esophagitis), which does not necessarily correlate with the presence or severity of symptoms.5 Lastly, effective therapy of GERD should prevent the complications of chronic reflux, including esophageal stricture, ulceration, and blood loss. Though intestinal metaplasia (Barrett's esophagus) is commonly regarded as a complication of GERD, there are no prospective endoscopic studies showing de novo development of Barrett's metaplasia in GERD patients who did not have it at initial endoscopy.
Lifestyle Modifications
Although the majority of patients with GERD require pharmacologic therapy, patients should be educated regarding the factors that contribute to gastroesophageal reflux and the nonpharmacologic measures that may improve symptoms (Table 1). Supine reflux is a common cause of nocturnal GERD symptoms, and as gravity plays a significant role in this entity, symptoms may be improved or eliminated by elevating the head of the patient's bed by at least 8 inches. This is best accomplished by the placement of wooden blocks or bricks under the bedposts. The use of a wedge that elevates the patient's head and thorax may also provide benefit but is not widely accepted; the use of multiple pillows elevates only the head and is ineffective in preventing reflux. Patients may find that sleeping with the left side down improves symptoms by augmenting the anatomic barrier to supine reflux. Patients should be advised not to lie down for at least 2 to 3 hours after a meal; this permits gastric emptying and reduces the quantity of gastric secretions available for reflux in to the esophagus. A recent study of patients with nighttime GERD symptoms reported that these practices provide "completely satisfactory" relief in approximately 50% of patients.1
Dietary modification may be helpful in improving reflux symptoms, and many patients can identify foods that induce or worsen their symptoms. Patients should be made aware of the most common provocative factors, including fatty foods, citrus fruits, tomato-based foods, coffee (including decaffeinated brands), chocolate, and alcohol. Patients should be advised to evaluate their own diets and eliminate the agents that precipitate symptoms; however, a blanket recommendation to avoid all food that may cause reflux is overly restrictive. Smoking is also associated with a higher frequency of symptomatic GERD, and the possibility of symptom relief may motivate smoking cessation efforts.
Many common antihypertensives, bronchodilators, and psychotropic agents promote gastroesophageal reflux, including calcium-channel blockers, nitrates, oral  2-agonists, and antidepressants with anticholinergic properties. A careful medication history is essential in evaluating GERD patients, as a simple medication change may greatly reduce symptoms.
2-agonists, and antidepressants with anticholinergic properties. A careful medication history is essential in evaluating GERD patients, as a simple medication change may greatly reduce symptoms.
Antacids
Antacids are a class of medications that act by directly neutralizing gastric acid. The use of antacids dates back to the ancient Greeks, who used ground coral powder (calcium carbonate) as a remedy for dyspepsia. Calcium carbonate (TumsR) is still a commonly used antacid, as is magnesium hydroxide (MylantaR, MaaloxR), and magnesium hydroxide with or without calcium (RolaidsR). Powdered sodium bicarbonate is also available, but is less frequently used. Antacids provide rapid, but temporary, relief of heartburn (lasting 30 to 60 minutes) and thus may require frequent dosing.
The few well-designed studies evaluating antacids in the treatment of GERD have generated conflicting results. Several trials have shown no significant difference between antacids and placebo in the control of heartburn,6 whereas others demonstrate a clear improvement of symptoms with antacid therapy.7, 8 No trials, however, have proven antacids to be effective in the healing of erosive esophagitis. Currently, antacid therapy is recommended for the treatment of GERD in patients with mild or infrequent symptoms.
Alginic acid, a polysaccharide derived from seaweed, may be used in combination with antacids (GavisconR) for the treatment of GERD. This agent creates a viscous layer atop the gastric juice and may impede acid reflux by physically preventing acid from entering the esophagus while delivering coadministered antacids to the esophagus. Antacid/alginic acid combinations have been demonstrated to be superior to placebo for the relief of GERD symptoms in small studies,9 and this therapy may also be used for the treatment of mild and infrequent heartburn.
Antacids are a very safe class of drug, but they are not without adverse effects, including diarrhea (with magnesium-containing formulations) or constipation (with aluminum-based formulations).
Prokinetic Agents
Gastroesophageal reflux is primarily a motility disorder, and the use of pharmacologic agents that improve esophageal and gastric motility are conceptually attractive as therapies for GERD. Unfortunately, the currently available prokinetic medications have only modest efficacy in relieving GERD symptoms, and the side effect profile of these agents renders them a less useful clinical practice.
Cisapride, a 5-hydroxytryptamine (5-HT4) receptor agonist that promotes the release of acetylcholine from the myenteric plexus, has been shown to be more effective than placebo in preventing heartburn10 and compared favorably with histamine-2 receptor antagonist (H2RA) therapy in small randomized trials.11 However, cisapride was significantly less effective than PPI therapy in controlling heartburn.12 Cisapride is no longer routinely available owing to its cardiac toxicity, with over 300 reported cases of cardiac arrhythmia and 80 patient deaths associated with its use.13 Cisapride can still be obtained under protocol on a limited basis for some patients who benefit from therapy or cotherapy with the drug. It is occasionally employed in severe diabetic gastroparesis.
Metoclopramide is a dopamine receptor antagonist that has been evaluated in the treatment of GERD in several trials. Most clinical studies of metoclopramide suffer from small sample size and are not placebo-controlled. The few well-designed studies demonstrate an improvement in symptom control over placebo using metoclopramide 10 mg po q.i.d.14 Metoclopramide is less effective, however, in both symptom control and healing of esophagitis when compared with H2RA therapy.15 Metoclopramide also has an unfavorable side-effect profile, which limits its widespread use. By crossing the blood–brain barrier, metoclopramide causes a number of central nervous system (CNS) effects, including drowsiness, agitation, and motor symptoms. These neurologic symptoms occur in up to 30% of patients and necessitate discontinuation of therapy. More serious side effects, including depression, precipitation of Parkinson's disease, dystonia, and tardive dyskinesia, are less common.13
Domperidone (MotiliumR) is a dopamine antagonist available from outside the United States or from sites within the U.S. via the Internet or Food and Drug Administration (FDA) referral. Unlike metoclopramide, domperidone does not cross the blood–brain barrier and thus has a better safety profile, with no significant CNS side effects and only a minor incidence of gynecomastia and galactorrhea. The effectiveness of domperidone in the treatment of GERD is not well established; a number of small studies have demonstrated inconsistent results with regard to the control of heartburn and mucosal healing.16 Small trials comparing ranitidine to domperidone found similar rates of symptom control and resolution of esophagitis, but demonstrated no added benefit with combination therapy using domperidone and an H2RA.15
Histamine–2 Receptor Antagonists
Histamine–2 receptor antagonists (H2RAs) bind to the histamine–2 receptor on the basolateral membrane of the gastric parietal cell. Histamine is secreted by the gastric enterochromaffin-like cell and is the principal stimulus for acid secretion; the inhibition of this paracrine effect significantly reduces gastric acid production. The first clinical studies evaluating H2RAs in the treatment of GERD demonstrated only modest benefit. These disappointing results were attributed to the fact that the dose of H2RA required to effectively treat duodenal ulcer is generally insufficient to treat GERD. Later trials utilized appropriate H2RA dosing and demonstrated a clinical effect significantly greater than placebo, both in the control of symptoms and the healing of esophagitis. Pooled results from clinical trials demonstrate a 50% to 75% rate of symptom control and mucosal healing.17 It should be noted that H2RA therapy is less effective in patients with severe erosive disease, with response rates of nearly 80% in grade I to II esophagitis, but only 30% to 50% in grade III to IV disease.9, 18, 19
The H2RAs are effective at inhibiting nocturnal acid secretion, and dosing of H2RAs either at bedtime or after the evening meal may provide effective nighttime relief, in patients with nonerosive disease or mild erosive esophagitis. Patients with moderate to severe esophagitis may require high doses twice daily.20 If given as a supplement to PPI therapy, only a small dose at bedtime is recommended, well separated in time from the evening dose of PPI. H2RAs have a relatively rapid onset of action, and are effective in treating episodic heartburn, an effect that is augmented by the coadministering of antacids. As a class of drugs, H2RAs are associated with a low incidence of adverse effects (<4%). Concern had been expressed regarding drug interactions owing to the effects of cimetidine (and to a lesser extent ranitidine) on the cytochrome P-450 system. Altered drug levels have been reported in patients using these early H2RA formulations, but these have not proven to be clinically relevant. The newer H2RAs, nizatidine and famotidine, have not been associated with significant drug interactions. Ranitidine is available in an intravenous formulation for use in the hospital setting, which may rarely be associated with delirium, especially in elderly patients.2
The development of pharmacologic tolerance to H2RAs has been demonstrated in many studies, and may occur after as little as 2 weeks of therapy.21 This phenomenon, known as tachyphylaxis, has not been shown in clinical trials to affect symptom control, mucosal healing, or maintenance therapy.
Proton Pump Inhibitors
Proton pump inhibitors are the most effective class of agents employed in the treatment of GERD. They covalently bind to and inactivate the H+/K+– adenosine triphosphatase (ATPase) enzyme located on the apical membrane of the gastric parietal cell, thereby blocking the final common pathway for gastric acid secretion. Proton pump inhibitors are prodrugs, which are absorbed from the small intestine, transported via the bloodstream to the gastric mucosa, and ultimately are secreted into the parietal cell secretory canaliculus. Owing to their pKa of 4 to 5, PPIs are inactive at the neutral pH of blood and of the parietal cell cytoplasm. After entering the secretory canaliculus of the stimulated parietal cell, with a pH of approximately 0.8 to 1.0, PPIs become activated via protonation. The resulting thiophilic sulfonamide covalently binds the H+/K+-ATPase, inactivating the proton pump and producing a profound decrease in acid secretion. It is important to recognize that PPIs bind only to activated proton pumps; thus, the optimal time to administer a PPI is prior to a meal, to ensure that drug is circulating during a period of parietal cell activation. Maximal pump activation occurs during the first meal of the day after an overnight fast, making breakfast the best time to administer a PPI in those with daytime or upright reflux; for those who do not eat breakfast, the once-a-day dose of drug should be taken 30 to 45 minutes before lunch. If symptoms are mainly nocturnal, it may be taken before the evening meal. When twice-daily dosing is required, the second dose should always be given before the evening meal, and not at bedtime.
The beneficial effect of PPIs in the treatment of reflux has been demonstrated in several studies, the majority of which compared PPI therapy to H2RAs. Two large trials with a total of 476 patients compared daily lansoprazole with ranitidine twice daily in patients with erosive esophagitis. Healing rates were >90% in the lansoprazole groups after 8 weeks, compared with 53% to 69% in the ranitidine groups.22, 23 A comparison of standard-dose lansoprazole (30 mg po qd) and high-dose ranitidine (300 mg po b.i.d.) demonstrated similar results, with 8-week healing rates of 91% and 66%, respectively.24 A large meta-analysis included 43 randomized controlled trials comparing PPIs to H2RA or placebo, and reported aggregate healing rates of 84% for PPIs vs. 52% for H2RAs.25 These data confirm the effectiveness of PPIs, which at standard doses appear to heal erosive disease in 85% to 95% of patients with GERD and esophagitis.
Head-to-head trials comparing the many available PPI formulations have demonstrated similar efficacy in the treatment of GERD. A meta-analysis of randomized controlled trials of PPIs in the treatment of erosive esophagitis reported no significant difference among omeprazole, lansoprazole, pantoprazole, and rabeprazole in control of symptoms or rates of mucosal healing.26 The most recent addition to the PPI class of drugs is esomeprazole, the S-enantiomer of omeprazole. Omeprazole, the first available PPI, is a racemic mixture of the stable S-enantiomer and rapidly metabolized R-enantiomer. Esomeprazole (40 mg) was compared to omeprazole (20 mg) in a large randomized controlled trial (n = 2425) of patients with erosive esophagitis.27 Esomeprazole at this dose was associated with a greater rate of mucosal healing and symptom control than omeprazole (93.7% vs. 84.2%). This is not unexpected, given the dose difference and that esomeprazole contains only the more potent enantiomer found in omeprazole. Esomeprazole does not, however, demonstrate clear superiority in comparison with other PPIs. A study of more than 5200 patients compared esomeprazole 40 mg po qd to lansoprazole 30 mg po qd in the treatment of erosive esophagitis. Healing rates with esomeprazole were 92.6% compared with 88.8% in the lansoprazole group.28 This difference was statistically significant, but the absolute rates of healing were very similar, underscoring the comparable clinical effectiveness of most PPI formulations. A different study comparing the efficacy of the same two drugs in relieving heartburn symptoms in 3034 symptomatic GERD patients over a 2-week period found lansoprazole to be marginally more effective than esomeprazole, but again the difference was clinically insignificant.29
Patients who do not respond to daily-dose PPI therapy should be questioned regarding the timing of therapy and should be encouraged to take their PPI before the first meal of the day. Barrison et al.20 reported that nearly 70% of primary care physicians prescribed PPIs to be taken before bedtime or without specific dosing instruction, suggesting that inadequate acid suppression with PPI therapy may be attributed in part to suboptimal administration. If symptoms do not improve, a second dose of PPI, to be taken before dinner, may be added. Patients who remain refractory to twice-daily PPI therapy should undergo esophageal ambulatory pH monitoring while on therapy to determine whether appropriate acid suppression has been achieved and whether the patient's symptoms are in fact a result of gastroesophageal reflux. Although the different PPI formulations have comparable efficacy, individual patients may experience idiosyncratic responses to different PPIs, and changing to another formulation may be indicated if inadequate acid suppression is documented on pH monitoring.
Refractory Gastroesophageal Reflux Disease
The term refractory GERD is used to describe patients who continue to have symptoms of gastroesophageal reflux despite conventional PPI therapy. This definition applies to symptoms and does not correlate with pharmacologic refractoriness, that is, failure of the drugs to suppress or abolish acid secretion. The term loosely used suffers from the additional limitation that it is meaningless unless twice-daily dosing with 40 mg of a PPI is specified; 40 mg of esomeprazole cannot be taken as equivalent to 10 or 20 mg of rabeprazole. This should be studied using ambulatory pH monitoring, but in the majority of those with refractory symptoms, inhibition of gastric acid secretion is achieved. True pharmacologic refractoriness is exceptionally rare and poorly studied. As described above, a minority of patients may respond symptomatically to an alternate PPI owing to patient-specific responses to different formulations. Patients who are truly refractory to twice-daily PPIs may have an acid hypersecretory state (e.g., Zollinger-Ellison syndrome) and a fasting serum gastrin level should be obtained after a 2-week period off PPI therapy. It is important that gastrin levels be drawn after a period offPPI therapy, as PPIs may elevate gastrin levels and reduce the specificity of the test. In patients who are diagnosed with gastrinoma, adequate control of acid secretion is often achieved only through the use of higher doses of PPI (up to 240 mg of drug daily).
Most patients with refractory GERD demonstrate appropriate acid suppression on ambulatory pH monitoring. Persistence of symptoms despite acid suppression raises the possibility of "functional heartburn," which may be a subset of patients with symptoms that mimic GERD. Alternatively, patients with persistent GERD symptoms may have an esophageal mucosa that is more sensitive to pH changes so that a less acidic refluxate still induces classic symptoms of reflux. Currently, acid reflux is defined as an esophageal pH <4 on pH monitoring; a pH of 5 or 6, however, remains acidic, and may be the cause of symptoms in a subset of patients with GERD. In such patients, higher doses of PPI may further decrease gastric acidity and relieve symptoms.30
Another possible cause of GERD symptoms despite effective acid suppression is bile acid reflux, in which duodenal contents mix with gastric secretions and come into contact with the esophageal mucosa. Bile acids in the esophagus are considered to be markers for the presence of small–bowel contents of uncertain composition; in the presence of acid, bile acids are protonated and therefore weakly acidic in themselves, perhaps augmenting the noxious effects of acid and pepsin.31 If acid suppression is effective, bile acids exist as neutral bile salts, which are less likely to induce mucosal injury. Whether or not bile acids or their salts contribute to refractory or conventional GERD remains uncertain. Refluxate containing bile is commonly found in patients who have undergone gastric surgery (i.e., subtotal gastrectomy with Billroth I or II anastomosis) for prior malignant disease or peptic ulcer. The contribution of such bile acid reflux to GERD, however, remains an area of active research. Tack's group32 recently examined patients with reflux symptoms refractory to daily PPI therapy. Esophageal acid exposure was determined using standard pH monitoring, and duodenogastroesophageal reflux was measured by a spectrophotometric method (Bilitec) that identifies bilirubin in the refluxate by measuring its characteristic light absorption spectrum. Of the 65 patients studied, 38% exhibited only bile acid reflux, with no pathologic acid exposure. Although these patients were not treated with maximal PPI therapy, the results of this trial suggest that reflux of intestinal contents, as indicated by the presence of bile, may indeed play a role in symptomatic GERD. Therapeutic options for such reflux are limited; aluminum hydroxide antacids and liquid sucralfate may bind bile acids, pancreatic enzymes, lysolecithin, or other molecules and offer some relief. Ursodeoxycholic acid is an orally available bile acid that is effective in the treatment of primary biliary cirrhosis and in selected patients with gallstone disease. It displaces more toxic bile acids in the bile salt pool and might decrease the noxious effects of duodenal reflux on the esophagus, but no clinical studies to date have demonstrated the efficacy of this agent in a clinical setting.
Maintenance Therapy
Gastroesophageal reflux disease is a chronic disorder, and the majority of patients relapse after discontinuation of antisecretory therapy. Chiba and colleagues33 performed a meta-analysis of clinical trials using PPIs and H2RAs in the treatment of GERD with esophagitis; symptoms recurred in approximately 80% of patients within 1 year after the cessation of therapy, with the highest recurrence rates described in patients with grade III to IV esophagitis.34 Long-term therapy for GERD has been shown to be safe and effective, with maintenance omeprazole therapy sustaining remission for up to 11 years in one long-term study, although a number of patients required transient PPI dose increases for short periods of symptomatic recurrence.35 Vigneri and colleagues36 compared the effectiveness of ranitidine, cisapride, omeprazole, ranitidine + cisapride, and omeprazole + cisapride for the maintenance of remission in patients with erosive esophagitis. All patients (n = 175) were initially treated with omeprazole for 4 to 8 weeks, and mucosal healing was documented by endoscopy. Patients were then randomized to one of the five therapy arms. At 12 months, remission was maintained in 80% of patients taking omeprazole, compared with 49% in the ranitidine arm and 54% in the cisapride arm. Omeprazole and cisapride in combination yielded the highest rate of remission (89%), but this was not significantly different from omeprazole alone. It is thus clear that PPIs provide the most effective therapy for the maintenance of remission in patients with reflux esophagitis. Recurrence of maintenance therapy in patients admitted to trials with heartburn—not just the 30% of heartburn patients who have endoscopic esophagitis—has not been adequately studied. In maintenance trials of those admitted with healed esophagitis, those randomized to placebo show 20% to 40% remaining symptom free during 1 to 2 years of follow-up.
"Step Therapy" in the Treatment of Gastroesophageal Reflux Disease
The concept of "step therapy" for GERD includes two approaches to treatment. "Step-up" therapy begins with lifestyle modifications, over-the-counter antacids, and/or low-dose H2RAs. If this is ineffective, the next step should include prescription H2RAs in full dosage (e.g., ranitidine 300 mg or famotidine 20 mg b.i.d.), with a final step up to PPI therapy if symptoms are not fully controlled. By contrast, "step-down" approaches initiate therapy with PPIs to provide the greatest chance of relieving symptoms. Therapy is then stepped down to H2RA therapy to determine whether relief of symptoms can be maintained with less potent acid inhibition. As stated above, it is uncommon for patients to be able to step down to nonpharmacologic therapy. These approaches have been evaluated in a small number of studies. Howden and colleagues37 randomized 593 patients with GERD to one of four treatment groups. Two groups of patients received either continuous ranitidine therapy (150 mg po b.i.d.) or continuous lansoprazole therapy (30 mg po qd). The two step-therapy arms received one of two regimens. One regimen consisted of ranitidine twice a day for 8 weeks followed by a step up to lansoprazole daily for 12 weeks; the other used lansoprazole daily for 8 weeks followed by a step down to ranitidine twice daily for 12 weeks. At the end of 20 weeks, the greatest degree of symptom control was reported in the continuous lansoprazole group.
In contrast, a study of step-down therapy by Inadomi and colleagues38 reported somewhat different results. In this study, 73 patients with GERD symptoms who had failed to respond to therapy with ranitidine 300 mg b.i.d. but who initially responded when stepped up to PPI therapy had their PPI dose decreased and then discontinued over a 4-week period. Patients who had recurrent symptoms off medication were treated with H2RA therapy twice daily with or without a promotility agent. At the end of 1 year, 58% of patients were no longer using PPIs; 27% of patients were off medication entirely, whereas 35% required H2RA therapy and 7% were on prokinetic agents. Younger patients and those with heartburn as a primary symptom were more likely to fail step-down therapy and require maintenance PPI therapy.
Although these studies of step therapy reached different conclusions, and although the Howden et al.37 study did not use ranitidine 300 mg b.i.d., the step-up approach has gained popularity in an era of managed care and cost controls. This not an unreasonable strategy, as many patients with nonerosive GERD or mild erosive esophagitis have adequate symptom relief with H2RA therapy alone. Patients with more severe disease who require a PPI may experience a delay in achieving symptom relief, and this needs to be balanced against the cost savings of any "step therapy" regimen.39
An Approach to the Treatment of Gastroesophageal Reflux Disease
The overwhelming majority of studies evaluating the treatment of GERD have included subjects with either documented erosive esophagitis or severe and frequent episodes of heartburn. Most individuals with GERD, however, have nonerosive disease and experience infrequent symptoms (up to three episodes per week). These patients may not have endoscopic evaluation prior to treatment, as this is typically reserved for patients with (1) long-standing symptoms (>5 years' duration) to exclude the possibility of Barrett's metaplasia; (2) "alarm symptoms," such as dysphagia, weight loss, or signs of gastrointestinal bleeding; or (3) onset of symptoms at an age greater than 45 years. The majority of patients, therefore, are treated on the basis of their symptom complex, rather than on endoscopic findings. Moreover, the presence or absence of esophagitis cannot be accurately predicted from a patient's symptom complex or from the response to therapy.5
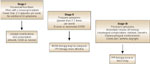
An approach to patients with symptomatic GERD is outlined in Figure 1. Gastroesophageal reflux is divided into discrete stages based on symptom frequency and severity, as well as the presence of esophageal complications or extraesophageal manifestations of GERD. Stage I GERD is defined as intermittent heartburn (up to three episodes per week) without complicating factors; this level of disease is effectively treated with lifestyle modifications, over-the-counter antacids, and/or nonprescription H2RAs. Stage II disease is characterized by more frequent symptoms (more than three times per week). Full dose therapy with an H2 blocker may be used initially, but PPI therapy is more effective in providing symptom relief and healing esophagitis, which may be present in this group of patients. Patients with stage III GERD have daily symptoms that remit as soon as antisecretory therapy is discontinued. Patients with GERD complications, including strictures and Barrett's esophagus, should be classified as stage III, as should patients with extraesophageal manifestations of GERD, such as asthma, laryngitis, or chest pain. These patients typically require PPI therapy either daily or twice daily to relieve symptoms and prevent complications. This staging system is widely applicable in clinical practice as it is based on presenting symptoms rather than the endoscopic finding of esophagitis; it also promotes graded therapy rather than an inflexible regimen of PPIs.
Figure 1: Approach to antisecretory therapy.
A suggested approach to the use of antisecretory therapy in the treatment of GERD, based on symptoms at presentation.
Nocturnal Gastroesophageal Reflux Disease Symptoms
Nighttime symptoms are common in patients with GERD, affecting a majority of those who report frequent daytime reflux symptoms. The importance of nighttime reflux has been underestimated, and a recent Gallup study reported significant morbidity related to nocturnal acid reflux. In a sample of 1000 Americans who reported GERD symptoms at least weekly, 79% reported nighttime symptoms of heartburn or regurgitation, and 63% noted that GERD symptoms hampered their quality of sleep. Moreover, 40% reported that their nighttime reflux symptoms and poor sleep had a negative effect on their daytime activities.1 In this cohort, 71% of patients reported using over-the-counter medications for their nocturnal symptoms, but only 29% considered this approach "completely satisfactory." Forty-one percent of patients in this group reported using prescription medications for their nocturnal GERD, and although 49% of these patients had complete symptom relief with this regimen, a full 51% remained dissatisfied with their symptom control. This study clearly demonstrates that nighttime symptoms of GERD are common and are often difficult to treat with current medical therapy.
Additional concern has been raised over the possibility that nocturnal reflux may be associated with a higher frequency of erosive esophagitis and GERD-related complications.40 A retrospective case-control study by Lagergren et al.41 demonstrated that symptomatic GERD was a significant risk factor for adenocarcinoma of the esophagus, with a relative risk of 7.7 in patients with recurrent symptomatic reflux. Patients with frequent nighttime symptoms had an even greater relative risk (approximately 11). Thus, nocturnal GERD symptoms have an important impact on the clinical sequelae of GERD.
Nocturnal Acid Breakthrough
Peghini and colleagues42 first described the concept of nocturnal acid breakthrough (NAB) in a study of patients with GERD who were taking PPIs twice daily. Ambulatory pH monitoring revealed that 73% of patients similar to normal controls, had a gastric pH below 4 for at least 1 hour during the night, a finding that has been confirmed in subsequent studies. The same authors also demonstrated that a single dose of an H2RA taken at bedtime reduced nocturnal gastric acid breakthrough in a study of normal volunteers.43
Further studies of nocturnal acid breakthrough, however, have had inconsistent results. Fackler and colleagues44 prospectively studied 40 subjects and found that the addition of an H2RA to twice daily PPI therapy was effective only during the first 24 hours after initiation of the H2 blocker. At 1 and 4 weeks, however, ambulatory pH monitoring demonstrated no difference in acid suppression between the two study arms. In contrast, Xue et al.45 retrospectively reviewed 105 patients on twice-daily PPI therapy (60 patients) or twice-daily PPI therapy combined with an H2RA at bedtime (45 patients). The percentage of time that gastric pH remained than 4 was 51% in the group taking PPIs alone compared with 96% in the group taking an additional H2RA. Esophageal acid exposure time was also decreased in the combination therapy group. Importantly, these pH studies were performed at least 4 weeks after initiation of H2RA therapy in a subset of patients, and the response to nighttime H2RA therapy was sustained.
A recent study prospectively compared four antisecretory regimens in a cohort of 22 subjects (13 with symptomatic GERD and nine normal volunteers).46 Patients underwent pretreatment pH monitoring and subsequently had repeat pH studies after each of four medical regimens: twice-daily PPI therapy (before breakfast and dinner), twice-daily PPI therapy plus an H2RA given at bedtime, PPI therapy given three times daily, and PPI therapy given before breakfast and at bedtime. Not surprisingly, the dosing of PPIs at bedtime, rather than before the evening meal, produced the least suppression of NAB (9%) compared with pretreatment pH studies. Twice-daily PPIs at mealtimes suppressed NAB in 18% of patients, and twice-daily PPI therapy combined with a nighttime dose of H2RA suppressed NAB in 41% of patients. This difference was not statistically significant, but the number of subjects in the study was small. It should be recognized, however, that NAB persisted in 59% to 91% of patients using any of these regimens, suggesting that none of these options is very effective at controlling nocturnal acid breakthrough. From these conflicting data, it can be concluded that nighttime H2RA therapy may be effective in patients with nocturnal GERD symptoms who are taking twice-daily PPIs. Larger prospective studies are needed to confirm this concept, but it remains a reasonable option for patients with seemingly refractory nocturnal GERD.
However, caution must be exercised when recommending an H2RA to patients on PPIs because concomitant administration of these two agents abolishes the acid inhibitory effect of PPIs.20, 38 Because H2RAs suppress parietal cell activation, the conversion of the inactive PPI prodrug to the active thiophilic sulfonamide may be retarded. Patients should accordingly be instructed never to take both agents together, and to take only small or modest doses of the H2RA at bedtime; higher doses of ranitidine (300 mg) or any dose of the longer acting famotidine (20 mg) will impair activation of a morning dose of any PPI.
Intermittent Therapy with Proton Pump Inhibitors
Proton pump inhibitor therapy for GERD is typically administered continuously, with either once-daily or twice-daily dosing. Such regimens have been conclusively demonstrated to control symptoms and heal erosive esophagitis. Moreover, as stated earlier, relapse rates after discontinuation of therapy are approximately 75%, confirming the need for maintenance or intermittent therapy. Maintenance PPI therapy has been proven effective in controlling symptoms over a 5- to 10-year period.34 Many patients, however, are rightly hesitant to commit to a lifelong regimen of daily antisecretory therapy. Recent studies have examined the possibility of intermittent therapy with PPIs, also known as "on-demand" therapy, in patients with nonerosive reflux disease or mild esophageal mucosal inflammation. Early studies of "weekend PPI therapy" given three days per week were disappointing, as symptom relief at 1 year was significantly lower with weekend therapy compared with daily dosing (32% vs 89%).47 Other studies, using alternate-day dosing regimens, published only in abstract form, have been more promising.
Lind and colleagues48 performed a large randomized controlled trial of on-demand therapy in 424 patients with nonerosive GERD. Patients with typical heartburn symptoms were treated for 4 to 8 weeks; those in whom symptoms resolved were randomized to receive omeprazole 20 mg, omeprazole 10 mg, or placebo. These medications were to be taken "on demand," such that if heartburn recurred, patients would use the study drug daily until symptoms resolved. The primary end point of this study was discontinuation of the protocol owing to poorly controlled heartburn. After 6 months, 83% of patients in the on-demand group taking omeprazole 20 mg remained on this regimen. In the lower dose omeprazole group, 69% remained on therapy, while only 56% continued therapy with placebo. The authors concluded that on-demand therapy may be effective in a substantial number of patients. It must be emphasized that this study assessed the effect of PPI therapy given on demand for periods of days to weeks; the use of PPIs on an "as-needed" basis (i.e., a single dose when symptoms occur) has not been proven effective.38 Talley et al.49 studied 342 patients with nonerosive GERD, using on-demand esomeprazole. After 6 months, 14% of patients discontinued the study medication owing to inadequate symptom control, compared with 51% receiving placebo. Proton pump inhibitor dosing during relapses in the on-demand esomeprazole group were as follows: 52% were treated for 1 to 3 consecutive days, 22% for 4 to 6 days, and 11% for 7 to 13 days. These two trials demonstrate that on-demand PPI therapy is well tolerated by patients who experience recurrent heartburn after discontinuing GERD therapy. This approach is less costly in terms of medication expenditures, but it remains to be determined whether patients outside of clinical trials will prefer interrupted symptom control to the complete relief obtained with daily maintenance therapy.
A recent review by Bardhan50 summarized the data from several randomized controlled trials and uncontrolled studies. He concludes that on-demand or intermittent PPI therapy is acceptable for younger patients with nonerosive or mild erosive GERD that responds rapidly to initial PPI therapy. Daily maintenance PPI therapy is preferred for patients with more severe esophagitis and those with a delayed response to PPI therapy. It should be noted, however, that on-demand therapy as defined in these trials is not in keeping with patients' conception of as-needed therapy. The goal of having rapid-onset long-lasting symptom control with a PPI taken for a single day is unrealistic given the pharmacokinetics of PPIs. In general, at least 4 to 5 days of continuous PPI therapy are required to maximally inhibit gastric acid secretion and ensure symptomatic benefit.39 It is unlikely that a patient using PPIs as needed will have adequate symptom relief unless the PPI is taken for several days at a time.
The Future of Pharmacological Therapy for Gastroesophageal Reflux Disease
Current research is focused both on improving antisecretory medications and in modifying gastrointestinal motility. Novel PPIs are in development, including a competitive inhibitor that reversibly binds near the K+ binding site of the H+/K+-ATPase.28 The clinical effectiveness of these so-called acid pump inhibitors compared with existing PPIs has yet to be determined. More interesting, however, is the ongoing research into non–acid-inhibiting agents that would improve pH restoration or esophageal clearance, enhance gastric accommodation or emptying, or perhaps reverse the disordered neuromuscular function underlying GERD. Baclofen, a GABAB receptor antagonist, has been reported to reduce the frequency of TLESRs in both healthy volunteers and patients with GERD.32, 51, 52 A randomized trial of baclofen therapy has yet to be performed, and will be necessary before GABABreceptor antagonists can be added to the armamentarium for the treatment of GERD.
The role of serotonin in the modulation of gastrointestinal motility is the subject of considerable research, and tegaserod, a selective 5HT4 receptor agonist, has been demonstrated to be effective in irritable bowel syndrome. Serotonin stimulates peristaltic contractions and increases gastric motility, and the use of serotonin agonists has the potential to ameliorate GERD symptoms by promoting gastric emptying and enhancing esophageal acid clearance. Early studies with tegaserod demonstrated a decrease in reflux episodes in treated patients,53 and larger controlled studies with this agent and other 5HT4 agonists are ongoing.
Other approaches to the treatment of GERD include inhibition of gastrin-mediated acid secretion with cholecystokinin antagonists, inhibition of histamine-mediated acid secretion via histamine-3 receptor blockade, and enhancement of esophageal mucosal protection with agents such as prostaglandin E2, epidermal growth factor, and transforming growth factor- . These remain in early phases of development and are not currently available for clinical use.
. These remain in early phases of development and are not currently available for clinical use.
Extraesophageal Manifestations of Gastroesophageal Reflux Disease
Acid reflux is responsible for a number of clinical syndromes outside the esophagus, and these extraesophageal manifestations of GERD have been recognized with increasing frequency (Table 2). As these symptoms may occur without coincident symptoms of heartburn and regurgitation, a high index of suspicion for GERD is required when patients present with any of these features. The association between GERD and pulmonary symptoms is well established. El-Serag and colleagues54 studied a cohort of over 100,000 service veterans with erosive esophagitis and demonstrated an increased association with asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis. Conversely, asthmatics have a higher prevalence of GERD symptoms than the general population, with nearly 80% of asthma patients reporting significant heartburn in some studies.17, 55 The role of acid reflux in adult-onset asthma in patients without classic GERD symptoms has been evaluated in a study by Harding et al.56 Ambulatory 2 pH monitoring was performed in patients with nocturnal asthma symptoms but no clinical evidence of GERD. Abnormal esophageal acid exposure was documented in 62% of this cohort.
Empiric PPI therapy in presumed GERD-induced asthma has been evaluated several small trials, with conflicting results. Kiljander and colleagues57 performed a randomized controlled trial in 52 patients with asthma and abnormal ambulatory pH studies. Omeprazole 40 mg twice daily for 8 weeks was associated with a significant improvement in nocturnal pulmonary symptoms; similar results were reported in an additional uncontrolled trial.56 Two other randomized trials, however, with a total of 50 patients reported no improvement in symptoms with PPI therapy.58, 59
Otolaryngeal symptoms, including hoarseness, globus sensation, and excessive throat clearing, may be manifestations of GERD. Koufman60 studied 225 patients presenting suspected GERD-induced otolaryngeal symptoms. Abnormal esophageal acid exposure was demonstrated in 62%, with pharyngeal reflux documented in 30%. Classic GERD symptoms were present in only 43% of study patients. Other trials suggest that nearly one third of otolaryngeal symptoms result from unsuspected GERD, and ear, nose, and throat physicians maintain a high index of suspicion for GERD in patients who are referred for hoarseness, chronic cough, or globus sensation.61, 62 Empiric PPI therapy in this patient population has been evaluated in several of small trials. El-Serag et al.,63 in a randomized controlled trial comparing lansoprazole 30 mg twice daily to placebo in 22 patients with reflux laryngitis, reported a 50% decrease in symptoms in the treated group, compared with 10% in the placebo group. Noordzij and colleagues,64 in a similar study, demonstrated a significant reduction in hoarseness and throat clearing in patients treated with omeprazole 40 mg twice daily for 8 weeks. Thus, for patients with suspected GERD-induced otolaryngeal symptoms, a trial of PPI therapy twice daily for 8 to 12 weeks is reasonable.
Noncardiac chest pain (NCCP) is defined as angina-type chest pain (substernal pressure or heaviness) occurring without demonstrable cardiac disease. It is not uncommon, with a prevalence of 23% reported in one study based in the northern United States.65 Although this syndrome is associated with several underlying conditions, including musculoskeletal pain and psychiatric illness, esophageal disorders are the most prevalent inciting factor. Of the esophageal causes of NCCP, GERD is the most common, accounting for 25 & to 60% of cases in several studies.66, 67 Other entities such as diffuse esophageal spasm, though often postulated as the cause of chest pain, are much less common. Given the absence of typical reflux symptoms in a subset of patients with NCCP, the diagnosis of GERD is usually established with ambulatory pH monitoring, which is abnormal in up to 60% of patients.68, 69 Several studies of PPI therapy in NCCP demonstrate improvement in symptoms with antisecretory therapy.70, 71 These observations have led to the use of empiric PPI therapy as a diagnostic test for reflux-induced NCCP. Proton pump inhibitor therapy is administered twice daily (before breakfast and dinner) for 8 to 12 weeks, and patients whose chest pain symptoms resolve are considered to have GERD as a cause of their NCCP. This PPI test has a sensitivity of approximately 80%, but patients who fail to respond to a therapeutic trial should undergo ambulatory pH monitoring to definitively rule out GERD as the etiology of chest pain.70