Key Points
- Oropharyngeal motor disorders can affect any of the three phases of swallowing and can result in dysphagia, aspiration, or both.
- Patients are best served through a team approach to care of oropharyngeal motor disorders.
- Failure of conservative management, concern for life-threatening aspiration, and long-term need for supplemental alimentation are indications for surgical management.
- Few disease state–specific treatments exist.
- Surgical interventions aim to protect the airway, ameliorate dysphagia, or provide alimentation by either correcting anatomic problems or bypassing affected steps in deglutition.
- Treatment of chronic aspiration can be categorized by voice sparing and voice sacrificing techniques as well as by reversibility.
- Current surgical therapy for dysphagia of oropharyngeal motor dysfunction origin is mainly limited to cricopharyngeal myotomy and surgical access for enteral feeding.
Introduction
A wide variety of conditions, including neurologic, muscular, and systemic disease, can present as oral and pharyngeal motor disorders (Table 1).1, 2, 3, 4, 5 In addition, the effects of aging, trauma, and intracranial as well as head and neck surgery can contribute to oral-pharyngeal motor dysfunction.
Briefly, there are three phases to swallowing: oral, pharyngeal, and esophageal. The oral phase is under voluntary control and is primarily concerned with initial bolus preparation and transport. Contact of the bolus against the posterior pharyngeal wall initiates the involuntary pharyngeal phase. Besides continued transport of the bolus, the pharyngeal phase is responsible for protection of the nasopharynx and larynx from soilage. Finally, the reflexive peristaltic waves of the esophageal phase propel the bolus into the stomach.6, 7
Motor disorders can affect any of the three phases and result in dysphagia, aspiration, or both; however, we limit our discussion to pathology related to oral-pharyngeal motor disorders. Surgical interventions aim to protect the airway, ameliorate dysphagia, or provide alimentation by either correcting anatomic problems or by bypassing affected steps in deglutition.
Although the focus of this review is on motor disorders, associated sensory deficits are important contributors to penetration and aspiration and are worth brief mention. Bastian and Riggs8 demonstrated that normal swallowing can occur spontaneously despite complete topical anesthesia of the upper aerodigestive tract (oral cavity, oropharynx, hypopharynx, larynx, and subglottis) with lidocaine. Nevertheless, recent studies have shown the laryngeal adductor reflex (LAR), defined as transient vocal fold closure after stimulation of the superior laryngeal nerve, ie stimulating the mucosa of the hypopharynx, to be an important step in preventing laryngeal penetration with swallow.9 Utilizing fiberoptic endoscopic evaluation of swallowing with sensory testing specifically aimed at assessing the LAR, patients with absent LARs were found to be at increased risk of aspirating thin liquids but not pureed foods.10, 11
General Principles
Patients are best served through a team approach to care of oropharyngeal motor disorders. The multidisciplinary team includes gastroenterologists, neurologists, otolaryngologist–head and neck surgeons, occupational and physical therapists, thoracic surgeons, and speech pathologists.12, 13 Rarely is surgery the primary treatment for motor disorders of the oral and pharyngeal area. Symptoms related to several of the neurologic causes of motor dysfunction, such as myasthenia gravis and Parkinson's disease, respond well to medical treatment.14, 15, 16 Speech pathologists and rehabilitation specialists, in addition to providing long-term care, can often help patients compensate for the physiologic changes related to their condition. Nonetheless, failure of conservative management, concern for life-threatening aspiration, and long-term need for supplemental alimentation are indications for surgical management.
It is difficult to categorize surgical options by disease state as there is considerable overlap in treatment. Likewise, a given condition, for example cerebrovascular accidents, can affect several anatomic sites, making a regional organizational scheme problematic. Instead, a clinical approach will be taken with surgical treatment options organized around the prevention of aspiration and dysphagia. Categorizing treatment in this way is somewhat arbitrary as patients may have both aspiration and dysphagia; however, a goal-oriented format facilitates discussion.
Surgical Treatment of Chronic Aspiration
Background
Aspiration is defined as entry of food or secretions into the airway below the level of the vocal cords.17 Symptoms may include coughing or choking during deglutition or, in more sinister cases, fever and purulent cough or outright signs of sepsis. In some cases patients may experience silent aspiration—no cough in response to aspiration—and report no symptoms.18, 19 Interestingly, scintography studies of normal subjects during sleep revealed a 45% aspiration rate.18, 19 However, further studies demonstrated that in normal subjects the average amount of material aspirated was on the order of 1.0 mm3.20
Numerous oropharyngeal motor disorders may lead to aspiration, but the treatment goal is uniform: to prevent further contamination of the airway. Continued significant aspiration can lead to bronchospasm, hypotension, hypoxia, and hypercarbia as well as fatal airway obstruction and pneumonia.
Initial Management
Before surgery is undertaken, initial patient management should include discontinuation of oral intake, institution of aggressive pulmonary toilet, appropriate antibiotics as indicated, and continued nutritional support either through an enteral or parenteral route. Enteral routes of alimentation include nasogastric tubes, cervical esophagotomy, gastrostomy, and jejunostomy. Surgical access for enteral feeding is discussed in a later section.
Indications
Failure of medical management along with the need for airway protection and prevention of aspiration pneumonia warrant consideration of surgical management. The ideal procedure would be effective, easily performed under local anesthesia, and allow continued phonation or deglutition while at the same time being potentially reversible should the patient's underlying process causing aspiration resolve.21 Unfortunately, no single procedure satisfies all these requirements, and so decisions must be based on the etiology of the condition, physiologic derangements, and patient prognosis and wishes.
Table 2 lists surgical treatment options for chronic aspiration according to reversibility and distinguishes whether postoperative phonation is possible.
Tracheotomy
Classic treatment for chronic aspiration, especially in neurologically debilitated patients and those requiring intubation, has been cuffed tracheotomy with our without gastrostomy. Tracheotomy can be performed rapidly, under local anesthesia, and is reversible. Although tracheostomy facilitates pulmonary toilet, it cannot be relied upon to prevent aspiration.22 In fact, tracheotomy itself has been associated with worsened aspiration through a variety of mechanisms as outlined in Table 3.23, 24, 25, 26, 27, 28 Additionally, the tracheotomy cuff does not protect the glottis but allows intermittent passage of material that has accumulated above the cuff.
Despite its limitations, tracheotomy remains an important part of the armamentarium for treating chronic aspiration. Several of the other surgical treatment options require tracheotomy as an adjunct, and many patients with oropharyngeal motor disorders, especially of neuromuscular etiology, may require tracheotomy for airway protection.
In addition to open tracheotomy, which has changed little since the time of Chevalier Jackson (a pioneering laryngologist in the late 19th century), percutaneous tracheotomy has become an increasingly popular option as it can be easily performed at the bedside under local anesthesia by both surgeons and nonsurgical intensivists. Several excellent descriptions of the techniques of open tracheostomy are available.29, 30
Currently, there are four major types of percutaneous tracheotomy: (1) Ciaglia's dilation over guidewire; (2) Grigg's modification employing guidewire dilating forceps; (3) Fantoni's translaryngeal tracheotomy, in which the tracheostomy tube is pulled from inside the trachea to outside at once without the need for serial dilation; and (4) the PercuTwist method, which utilizes a screw-in dilator. All of the techniques have been shown to be effective and reproducibly safe.31, 32, 33, 34, 35 At this time, the only commercially available kits for percutaneous tracheotomy in the United States are based on either Ciaglia's serial dilation technique or a modified technique with a single dilator. (References to the techniques with information on the international kits are given above.)
Complications
Complications resulting from tracheotomy can be classified on a temporal basis as intraoperative, early postoperative, and late postoperative. Table 4 lists complications by period.30, 36 Though there may be overlap in the time frame between the early and late postoperative periods, this scheme focuses on the approximate time period where a given complication is likely to occur. Bleeding (3.7%) has classically been the most common complication, followed by tube obstruction (2.7%), and tube displacement (1.5%). The dread complications of pneumothorax, tracheal stenosis, and tracheoesophageal fistula occur less than 1% of the time. Death, which occurs in 0.5% to 1.6% of cases, is most often caused by hemorrhage or tube displacement.29, 30 In one of the largest series of consecutive tracheotomies compiled, there was a 4.3% major complication rate with eight deaths after 1130 consecutive tracheotomies.36 As would be expected, the likelihood of complications is increased in emergent tracheotomies as compared to elective procedures.
Multiple studies comparing the complication rates of open and percutaneous tracheotomy have yet to establish one procedure as clearly superior.37, 38, 39 Rather than a flaw in study designs it is likely that the equivocal results indicate that both techniques are comparable in terms of safety and efficacy. Interestingly, a well-designed prospective randomized controlled trial comparing bedside open surgical tracheostomy with endoscopically guided percutaneous tracheotomy found no difference in perioperative complication rates, but percutaneous tracheotomy resulted in a higher incidence of postoperative complications (16% vs. 2%, p <.05) and was actually less cost-effective.40 A similar prospective randomized controlled study from Taiwan found percutaneous tracheotomy to have significantly reduced operating time while maintaining comparable complication rates compared to the open tracheotomy control.41
Endolaryngeal Stent
Endolaryngeal stents, commonly used for laryngotracheal support, represent a relatively easy, potentially reversible procedure for the treatment of chronic aspiration for up to a year.42, 43, 44, 45 In addition to ease of placement and reversibility, laryngeal stents used to obturate the larynx have the benefits of minimal incisions (none if a tracheotomy is already in place) and dissection, short operative time, and, if unsuccessful, endolaryngeal stent removal does not hinder other procedures for treatment of aspiration.
Two types of endolaryngeal stents have been described, both of which also require tracheostomy. Weisberger and Huebsch44 utilized endoscopically placed Silastic stents to control aspiration. Subsequently, Eliachar and Nguyen46 developed a similar laryngeal stent that incorporated a one-way valve above the level of the true vocal cords that, unlike the Weisberger stent, allowed phonation.
Weisberger and Huebsch Laryngeal Stent
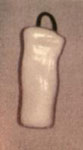
In 1982, Weisberger and Huebsch44 reported the results of endoscopic treatment of aspiration in seven patients using a Silastic laryngeal stent (Figure 1). Under endoscopic guidance, three percutaneous sutures were passed into the tracheal lumen. One suture was used as a guide to transorally place the stent in its desired location while the other two sutures were used to secure the stent in place.
Figure 1: Weisberger and Huebsch endolaryngeal stent (Montgomery's stent) for treatment of chronic aspiration.
(Source: From www.emedicine.com, with permission from John E McClay, M.D.)
Postoperatively, the majority of patients had decreased aspiration and improvement in pulmonary complications related to aspiration. However, the authors noted concern over the high postoperative mortality rate with four of the seven patients treated eventually succumbing. Two of the four deaths were thought to be related to tracheotomy tube obstruction and the other two were related to the patient's underlying medical problems.
One of the reported benefits of endolaryngeal stents is their potential reversibility. In this study, endoscopic removal of the laryngeal stent was performed in two patients but persistent aspiration prompted replacement of the stents. The authors cite 1 year as the upper limit for stent placement and so with continued need for the stents beyond that time frame, either a new stent or a different technique must be employed.
Eliachar and Nguyen Laryngeal Stent
The Weisberger and Huebsch stent had several of the characteristics of the ideal stent—a relatively simple procedure, effective, and reversible—but patients were no longer able to phonate. Eliachar and Nguyen46 subsequently reported the use of a laryngotracheal stent placed under rigid bronchoscopic guidance that allowed for continued phonation. The presence of a domed one-way valve that rises above the level of the vocal cords in the Eliachar stent permits air to escape from the lungs but blocks passage of materials beyond the glottis. Another difference is a posterior skirt-like projection that extends inferiorly to support the posterior tracheal wall down to the level of the upper convex surface of the tracheostomy tube (Figure 2).
Figure 2: Eliachar endolaryngeal stent for treatment of chronic aspiration while allowing phonation.
(Source:Hood Laboratories, Pembroke, MA., with permission)
Similar to the Weisberger stent, endoscopic guidance is used. Here a rigid bronchoscope is used to aid in placement of the stent. Instead of transoral placement, the Eliachar stent is inserted through the tracheostomy site and guided cranially under visualization from the bronchoscope until the superior-most portion of the stent is approximately 1 cm above the vocal cords. In its final position, a strap extends caudally from the anterior wall of the stent and exits through the tracheostomy site riding along the upper convexity of the stoma.
All 12 patients who underwent stent placement for control of aspiration had resolution of their aspiration except for one patient who required placement of a larger stent before aspiration was controlled. Significant postoperative mortality was also seen with this stent, with nine of the 12 patients dying. Reportedly, death was related to their primary disease and not continued aspiration. Stents were kept in place for as long as 9 months, and eventual stent removal was achieved in all three of the survivors. One of the survivors, however, required laser surgery for granulation tissue and another had anterior subglottic webbing at 1 year post–stent removal but did not require surgery.
Complications
Complications from endolaryngeal stent placement include the risks inherent in nearly all surgical procedures, namely bleeding and infection as well as the previously described complications associated with tracheotomy. Postoperative complications are also similar to those of laryngeal stent placement for internal support and include tracheal stenosis, formation of granulation tissue, and stent migration or failure. High postoperative mortality rates were seen in both studies, but this was more likely a reflection of the critically ill status of the patients rather than a result of complications directly related to the procedure. A clear limitation that both studies delineate is the purely temporary role of the stents and the need for alternate management should aspiration continue beyond the span of months. Patient discomfort, glottic granulation, and the potential difficulties with appropriate sizing have limited the use of the stents.47
Epiglottic Flap Laryngeal Closure
Supraglottic laryngeal closure prevents aspiration by blocking off the entrance to the glottis and by facilitating transport of the bolus down the lateral pharyngeal channels. Supraglottic closure requires tracheotomy but allows for potential postoperative phonation and reversibility. Several methods of supraglottic closure have been developed based on Habal and Murray's48 initial case report of an epiglottic flap to the arytenoids for laryngeal closure. The patient was a 9-year-old girl who had underwent posterior fossa exploration of a papillary ependymoma and postoperatively remained gastrostomy and tracheotomy dependent with pulmonary complications from aspiration. Given the patient's age and hope for some recovery of function, Habal and Murray designed their epiglottic-arytenoid flap as a reversible means of protecting the larynx.
A suprahyoid skin incision was used to gain access to the hypopharynx above the level of the glottis. The epiglottis was retracted anteriorly and after freshening the edges, the epiglottis was brought posteriorly to cover the glottic inlet and sewn to the denuded aryepiglottic folds. A second layer composed of bilateral rotational flaps of mucosa from the pyriform sinuses was used to cover the raw surface of the epiglottis. Follow-up 1½ years later revealed the patient to still be tracheotomy dependent but tolerating a regular diet and with the ability to phonate owing to the fortuitous creation of a one-way valve by a small posterior dehiscence in the epiglottic-arytenoid flap.
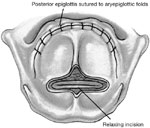
Strome and Fried49 added steps to decrease the likelihood of dehiscence by relaxing tension on the epiglottic flap by severing the hypoepiglottic and thyroepiglottic ligaments. Morselization, linear striation, or small wedge resections were also used to decrease the tensile strength and elasticity of the epiglottis (Figure 3).
Figure 3: Supraglottic closure with epiglottic flap incorporating relaxing incision of epiglottis.
(Source: Wisdom et al.47, with permission)
Strome and Fried also stressed the need for preoperative visualization of the epiglottis to ensure that its dimensions were adequate for laryngeal closure and was the first to report endoscopic sequential reversal of the flap. Under suspension, laryngoscopy microlaryngeal instruments or a laser can be used to create an opening of the desired size in the epiglottic flap to allow phonation. The creation of a 30% opening in the flap allowed a patient, reluctant to undergo complete reversal, to phonate while continuing to prevent aspiration. Other groups, have instead intentionally left the posterior laryngeal inlet open to allow phonation.50, 51
A second layer of supraglottic closure was incorporated in Castellanos's52 series of seven patients with chronic aspiration treated by transthyrotomy petiole supraglottopexy. The distinguishing feature of his modifications was that in addition to epiglottic flap closure of the supraglottis, a second inferior closure was made by incising and then approximating the false vocal cords. Of the seven patients who underwent this procedure, all were subsequently able to tolerate at least some oral feeding, and only one patient had evidence of flap dehiscence. Owing to the nature of the procedure, postoperative phonation was not possible. However, as the true vocal folds were left intact with correction of the underlying condition leading to aspiration, reversal could be performed to allow phonation.
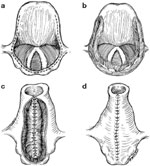
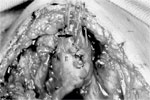
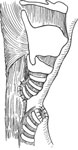
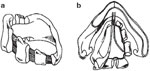
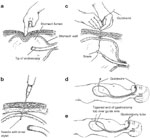
Biller et al.'s53 tubed supraglottic laryngoplasty represents a further departure from Habal and Murray's original epiglottic flap. A lateral pharyngotomy or approach similar to glossectomy is used to access the supraglottic larynx. To raise the tube-shaped flap, an incision is made along the lateral borders of the epiglottis continuing on to the aryepiglottic folds and across the arytenoids mucosa and then posteriorly in the interarytenoid mucosa (Figure 4a). The mucosal flaps are then elevated forming two layers of closure—a deeper layer formed by the medial edges of the flap and a superficial layer formed by the lateral edges of the flap (Figure 4b). The resultant closure is a chimney-shaped structure composed of the supraglottic structures (Figure 4c,d). A small opening at the superior aspect of the tube allows for phonation while the height of the tube protects the posterior commissure and posterior aryepiglottic space from airway soiling. Figure 5 shows an intraoperative view of the larynx after closure of the supraglottis.
Figure 4: Biller's tube supraglottic laryngoplasty for supraglottic closure.
a: Incision along lateral border of epiglottis marked out. b: Mucosal flaps elevated. c: Closure of deep layer of mucosal flaps. d: Final closure with small opening at superior aspect of tube to allow for phonation. (Source: Pletcher and Eisele.58, with permission)
Figure 5: Intraoperative photograph of tubed supraglottic laryngoplasty.
The arrow indicates the location of the opening in the closure to allow phonation. E, epiglottis. (Source: Sato and Nakashima.54, with permission)
More recently, a Japanese group, responding to reports of occasional leakage of the posterior part of the tubed laryngeal closure, modified Biller's technique to include arytenoid and interarytenoid mucosal flaps for closure of the posterior glottis prior to supraglottic closure with the arytenoids and aryepiglottic folds.54 The three patients who underwent this procedure all had their respiration and deglutition restored without aspiration and were demonstrated to have complete closure of the posterior part of the larynx.
Complications
Though no large-scale studies of epiglottic flap closure of the larynx have been performed, success rates have been reported to be between 50% and 75%.21, 55, 56 The most frequently cited complication has been flap dehiscence, especially along the posterior portion of the flap.6, 21, 48, 49, 55, 56 On some occasions this has been welcomed as a one-way valve to allow phonation, whereas other cases required reoperation to prevent further aspiration.48, 55 By design, the procedure is reversible and although there are few reports of successful reversal in the literature, supraglottic stenosis would certainly be a potential complication after reversal.52
Tracheoesophageal Diversion and Laryngotracheal Separation
Thus far, the procedures described have relied on improved pulmonary toilet (tracheotomy) or prevention of continued transit of material down the shared alimentary and respiratory tract into the trachea (cuffed tracheotomy, endolaryngeal stents, and supraglottic closure). More definitive techniques include the complete separation of the alimentary and respiratory passages and include partial and subperichondrial cricoidectomy, narrow-field laryngectomy, tracheoesophageal diversion, and laryngotracheal separation. Of these, only the related techniques of tracheoesophageal diversion and laryngotracheal separation are reversible.
In recent years, subglottic closure utilizing either of these two methods has become the preferred choice in surgical management of intractable aspiration.57 Both procedures have the advantages of being potential bedside procedures, allowing oral alimentation, and upon reversal allowing return to preoperative phonation as the endolarynx and larynx are not injured.58 Though normal air-powered speech is not possible, neurologically intact patients may use an electrolarynx. Additionally, transesophageal puncture and placement of a Blom-Singer voice prosthesis allow esophageal speech.59
Tracheoesophageal Diversion
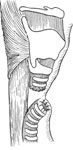
Lindeman60 first developed the technique of tracheoesophageal diversion on mongrel dogs and demonstrated its reversibility before attempting the procedure on an elderly woman with pharyngeal and laryngeal motor dysfunction and chronic aspiration secondary to lower cranial nerve damage after resection of a large vestibular schwannoma. A midline incision below the level of the cricoid cartilage was used to expose the trachea with careful lateral retraction of the recurrent laryngeal nerves. The trachea was completely transected between the third and fourth rings. An end-to-side tracheoesophageal anastomosis was performed utilizing the proximal tracheal segment and anterior cervical esophagus. The distal tracheal was brought out to the skin as a tracheotomy as would be done in a total laryngectomy (Figure 6). At follow-up 5 months later, the patient's laryngeal mucosa appeared normal despite the passage of food and saliva through the larynx for several months.
Figure 6: Tracheoesophageal diversion for subglottic closure and complete separation of the alimentary and respiratory passages.
(Source: Pletcher and Eisele.58 with permission)
Laryngotracheal Separation
In Lindeman's original case report, the patient did not initially have a tracheotomy in place. However, in many cases by the time surgical consultation is obtained for definitive management of chronic aspiration a traditional high tracheotomy (between the second and third tracheal rings) will have already been performed. High tracheotomies make the end-to-side tracheoesophageal anastomosis difficult by limiting movement of the proximal tracheal segment. To address this, Lindeman et al.61 modified the original procedure by oversewing the proximal tracheal stump in layers and reinforcing the closure with rotated sternothyroid muscle flaps (Figure 7). The resultant laryngotracheal separation obviated the need for an esophageal anastomosis but left a blind proximal tracheal pouch instead.
Figure 7: Laryngotracheal separation obviating the need for esophageal anastomosis by closure of the proximal tracheal stump.
(Source: Pletcher and Eisele.58 with permission)
Although no complications related to the blind pouch were reported in Lindeman's report, and, in fact, it is difficult to find mention of complications owing to the proximal tracheal stump in the literature, food and saliva will inevitably fill the stump. Nearly a decade later, Krespi et al.62 described a procedure that permitted tracheoesophageal diversion despite high tracheotomy by allowing increased mobility of the proximal tracheal segment through resection of the proximal anterior tracheal cartilages and the inferior half of the cricoid cartilage. The resultant superiorly based tracheal mucosal flap is then anastomosed to an anterior, horizontal esophagotomy to effect the diversion (Figure 8). In Krespi et al.'s small series, all five patients had resolution of their aspiration and four were able to tolerate oral alimentation postoperatively. Other modifications, such as directing the proximal tracheal stump out the lateral neck through a split in the sternocleidomastoid muscle have not been as well received.63
Figure 8: Modified tracheoesophageal diversion allowing esophageal anastomosis despite high tracheostomy.
Increased mobility of the proximal tracheal segment through resection of tracheal cartilage allowed for tracheal anastomosis to an anterior, horizontal esophagotomy. (Source: Wisdom et al.47, with permission)
One of the largest reported series of laryngotracheal separation for chronic aspiration is from Pittsburgh. Thirty-four patients were operated on over a 9-year period. The majority of patients underwent laryngotracheal separation as a result of neurologic causes of oropharyngeal or laryngeal dysfunction resulting in aspiration, with the remaining patients having symptoms related to head and neck surgery. Aspiration was relieved in all patients, although several remained unable to swallow owing to their underlying neurologic condition. All patients were unable to tolerate oral feeding prior to surgery, while after surgery nearly one third were able to tolerate a regular diet. A little over one half of the patients continued to require supplemental feeding through a nasogastric tube or gastrostomy.
Two patients subsequently underwent tracheal reconstruction. One patient had resolution of a multiple sclerosis exacerbation and demonstrated improvement in her neurologic status. After reconstruction she was decannulated and had normal deglutition. The second patient was reconstructed after suboccipital decompression of his brainstem and vagal nerves owing to platybasia caused by osteogenesis imperfecta. Following reconstruction, stenosis developed at the tracheal anastomosis site; however, following revision the patient was able to speak and swallow with a tracheotomy tube in place.
Complications
Tracheoesophageal diversion and laryngotracheal separation are relatively straightforward procedures that may even be performed under local anesthesia. Few serious complications have been reported. Postoperative fistula has been the major concern following these procedures.47, 58 Fortunately, most fistulas resolve with conservative management and do not greatly affect outcomes. In the Pittsburgh series greater than one third of the patients who underwent laryngotracheal separation developed postoperative fistulas. However, only one patient required reoperation, and surprisingly fistula formation was not associated with longer hospital stays.57 It was noted that fistula formation was more likely in patients who necessitated muscle flap closure of the proximal trachea and in those patients with prior tracheostomies, indicating that the short proximal stump and adhesions may have made a tension-free mucosal closure technically more difficult.
Partial Cricoidectomy
All of the previously discussed surgical options for intractable aspiration have been reversible. There are several procedures without the potential for reversal that are useful treatments for chronic aspiration when there is no hope for return of function or improvement in the fundamental condition causing symptoms. Of the irreversible procedures, only partial cricoidectomy permits continued phonation (Table 2). Krespi et al.64 first described partial cricoidectomy for the treatment of aspiration in patients who had undergone major head and neck resections involving the base of tongue, pharynx, or larynx.
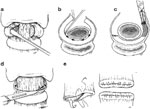
A lateral approach is used to gain access to the posterior larynx. The posterior attachments of the thyroid cartilage are cut and the thyroid cartilage is rotated away to one side like a hinged door to approach the posterior cricoid cartilage. The posterior cricoid perichondrium is elevated and the posterior half of the cricoid lamina is carefully removed with small rongeurs, being careful not to enter the mucosa or injury the adjacent cricoarytenoid joints, which could affect speech (Figure 9). Concurrently a cricopharyngeal and inferior constrictor myotomy is performed. The goal of these maneuvers is to create a larger hypopharyngeal inlet to facilitate swallowing while at the same time decreasing the diameter of the laryngeal inlet to help prevent aspiration. Tracheotomy was a necessary adjunct due to airway collapse.
Figure 9: Appearance of larynx after posterior cricoid resection.
(Source: Wisdom et al.47, with permission)
Instead of utilizing a lateral cervical approach to the cricoid lamina risking injury to the recurrent laryngeal nerve and cricoarytenoid muscle, Mendelsohn56 promulgated a translaryngeal approach with laryngofissure. This minimized exposure of the recurrent laryngeal nerve and permitted enough lateral cricoid cartilage to remain to prevent airway collapse. The patient in the case report had tolerated removal of tracheostomy and gastrostomy well after 12 months of follow-up.
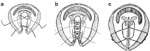
Around the same time as Krespi's report, Biller and Urken65 reported the results of a similar procedure. Vertical segments along half of the cricoid cartilage were removed collapsing it medially to narrow the laryngeal inlet and correct glottic incompetence (Figure 10).
Figure 10: Partial cricoid resection modification of posterior cricoid resection.
Vertical segments along one half of the cricoid cartilage are removed collapsing the remaining cricoid cartilage medially narrowing the laryngeal inlet. a: Oblique view of cricoid. b: Superior view of thyroid cartilage and cricoid. (Source: Biller and Urken.65, with permission)
Complications
There are no large reported series of partial cricoidectomy. In Krespi's original report, submucosal, subtotal, posterior cricoid resection was performed in nine patients to facilitate swallowing and reduce aspiration. Six of the nine were subsequently able to tolerate an oral diet, and all patients were able to effectively manage their secretions, though no patients were decannulated. No complications were reported, although it was noted that injury to the posterior cricoarytenoid muscle or its nerve supply at the site of surgery may be unavoidable.64 Biller and Urken65 performed cricoid collapse in four patients, with three of the four patients able to swallow without aspiration after surgery. Complications from the surgery were not mentioned.
Subperichondrial Cricoidectomy
When recovery of neuromuscular function of the upper aerodigestive tract is not expected, especially with preoperative loss of phonation and laryngeal protective function, subperichondrial cricoidectomy may be the procedure of choice for definitive separation of the upper alimentary and respiratory passages. Subperichondrial cricoidectomy has similar success rates as other irreversible procedures, such as narrow-field laryngectomy and glottic closure, but is a simpler procedure with low morbidity.21, 58
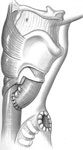
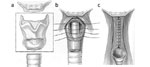
The procedure begins with a cervical vertical midline incision and dissection to expose the anterior cricoid cartilage, which is divided in the midline. The outer and inner perichondrium are elevated circumferentially and the cricoid is removed piecemeal with biting forceps. The inner perichondrium and mucosa are transected, inverted, and closed forming a subglottic pouch. The outer perichondrium forms a second layer of closure, incorporating a rotated sternohyoid muscle flap intercalated between the subglottic pouch and the necessary tracheostomy (Figure 11).66
Figure 11: Subperichondrial cricoidectomy for definitive separation of the upper alimentary and respiratory passages.
a: Elevation of outer cricoid perichondrium. b: Circumferential elevation of inner cricoid perichondrium. c: Anterior cricoid cartilage removed with biting forceps leaving the posterior lamina intact. d: Inner perichondrium and mucosa are transected. e: Closure forming a subglottic pouch. (Source: Modified from Eisele et al.66, with permission)
Complications
Eisele et al.66 reported the results of superperichondrial cricoidectomy for chronic aspiration and pulmonary complications in four patients. The procedure produced definitive separation of the respiratory and digestive tract and complete control of aspiration in all patients. No complications were reported; however, initial development of the procedure was complicated by fistula formation of the subglottic mucosa until changes were made in the closure.
Glottic Closure
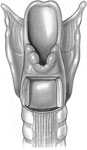
We have discussed both supraglottic and subglottic closure procedures, and so it should come as no surprise that glottic closure should be another option for surgical treatment of chronic aspiration. Montgomery67, 68 was the first to describe a method of glottic closure for protection of the subglottic airway. Through a transcervical approach a thyrotomy is performed to gain access to the endolarynx. The mucosa of the true and false vocal folds are denuded and closed in two separate layers with permanent sutures (Figure 12). A third layer of closure, consisting of a superiorly based sternohyoid muscle rotational flap, may be incorporated to help prevent fistula formation.69 Permanent tracheostomy is a necessary adjunct to this procedure.
Figure 12: Glottic closure for treatment of chronic aspiration.
a,b: The denuded mucosa of the true and false vocal cords are closed in two layers. c: Appearance of final closure. (Source: Pletcher and Eisele.58, with permission)
Glottic closure by design sacrifices normal speech because the vocal cords are fixed together. In fact, preoperative vocal cord paralysis or weakness may be beneficial as continued laryngopharyngeal movement may place excessive tension on the closure. Nevertheless, the success rates for glottic closure have been excellent—around 93%.21, 67, 68, 69 We have chosen to classify glottic closure as being irreversible despite the fact that reversal is technically possible. Though the normal anatomic relationships of the laryngeal structures may be restored, the damage to the true vocal fold mucosa and musculature are unlikely to be reversed. Additionally, the body of experience with repair of glottic webbing (which would be expected after long-term glottic closure) from intubation injury or other laryngeal trauma would indicate that successful reversal of glottic closure indeed would be very difficult.47 This has been borne out in the literature, with only a single successful report of glottic closure.70
Complications
Montgomery and Sasaki treated a total of 17 patients with glottic closure and had uniformly good results with no reported complications. Sasaki noted that with Montgomery's original technique dehiscence of the closure lines may result in continued aspiration and so added a third layer of closure. At the 6-month follow-up, all five treated patients had complete glottic closure.
Narrow-Field Laryngectomy
Prior to Habal and Murray's48 laryngeal closure procedure in 1972, total laryngectomy had been the definitive surgical treatment for chronic aspiration unresponsive to medical management. Similar to subperichondrial cricoidectomy, laryngectomy is considered in cases where the pathology causing aspiration is likely to persist and there is no hope for return of laryngeal protective function. Laryngectomy, however, is a more involved procedure and adds additional psychosocial stressors with the loss of the voice box and cosmetic deformity with the loss of the laryngeal prominence.71, 72 A recent study comparing tracheoesophageal diversion to total laryngectomy for chronic aspiration found both techniques to have similar results and complication rates; however, total laryngectomy resulted in greater surgical stress evidenced by greater blood loss and longer operative times.73
Currently, narrow-field laryngectomy, in which the hyoid, strap muscles, and as much pharyngeal mucosa as possible are spared, is preferred to total laryngectomy for treatment of intractable aspiration (Figure 13).
Figure 13: Narrow-field laryngectomy for definitive separation of the upper alimentary and respiratory passages.
a: Area to be resected within dotted box. b: Pharyngeal mucosa spared. c: Strap muscles closure forming second layer over pharyngeal closure. (Source: Wisdom et al.47, with permission)
Complications
Based on a study of 203 patients who underwent laryngectomy for malignancy, the most common significant postoperative complications were pharyngocutaneous fistula (28.5%), wound infection (28%), pharyngeal stenosis (6%), and flap necrosis (<1%).74 Meticulous technique and closure without tension are critical to prevent a pharyngocutaneous fistula. Narrow-field laryngectomy for chronic aspiration should allow for greater conservation of pharyngeal mucosa compared to resections for malignancy, and so pharyngocutaneous fistula would be expected to be a less frequent occurrence.
Surgical Treatment of Dysphagia
Background
Oropharyngeal dysphagia, difficulty in effective passage of solids or liquids from the oropharynx into the upper esophagus, can result in chronic cough, fluctuating hoarseness, recurrent pulmonary infections, and malnutrition. The management objectives of dysphagia are threefold: (1) identification of factors that cause or aggravate swallowing dysfunction, (2) assessment of dysphagia severity, and (3) institution of an appropriately individualized management plan.75
A comprehensive evaluation, in addition to a detailed history and physical exam, should include both endoscopic and radiographic examinations such as fiberoptic laryngoscopy and modified barium swallow76, 77 as well as laboratory tests as indicated. Assessment of possible causative factors may reveal a condition responsive to medical management. For example, both myasthenia gravis and Parkinson's disease are neurologic conditions known to cause dysphagia through their effect on the pharyngeal musculature and both conditions have been shown to be responsive to pharmacotherapy.14, 15, 16
The severity of the dysphagia is critical in determining the timing, mode, and extent of treatment. Individualized treatment plans are important as certain conditions are more amenable to surgery, such as structural cricopharyngeal disorders.78 In general, unfortunately, results of surgery for the correction of dysphagia have been less than promising. A plethora of conditions may cause dysphagia; however, we limit our attention to treatment of diseases that result in oropharyngeal motor dysfunction (Table 1).
Initial Management
Just as in the treatment of aspiration, management of dysphagia owing to oropharyngeal motor dysfunction falls within the domain of several specialties. Many institutions have adopted a team approach with representatives from gastroenterology, neurology, otolaryngology, radiology, and speech–language pathology.
Initial nonsurgical management centers on dietary modification, swallow therapy, and, when possible, treatment of the underlying medical condition resulting in dysphagia. Dehydration and poor intake can initially be treated with intravenous hydration and nasogastric enteral feeding.79
Indications
Surgical intervention should be entertained after more conservative treatments have proven unsuccessful. As discussed in the previous section, dysphagia leading to life-threatening aspiration is an indication for surgery. Additionally, protein energy malnutrition secondary to dysphagia may require surgical access for enteral feeding. Treatment of oropharyngeal motor disorders aims to correct or compensate for the underlying physiologic defects.
Cricopharygeal Myotomy
Cricopharyngeal myotomy is the only surgical means of improving bolus transport and has become the most common surgical treatment for oropharyngeal dysphagia.80, 81 The cricopharyngeus muscle, named by Antonius Maria Valsalva in 1717, is situated between the hypopharynx and esophagus and forms the upper esophageal sphincter, which separates the pharynx from the esophagus.82 The cricopharyngeus muscle extends from the anterolateral aspect of the cricoid cartilage continuously to the opposite cricoid cartilage. Studies of surgical and autopsy specimens have shown the vertical height of the muscle averages nearly 2 cm.83 At rest, upper esophageal sphincter pressure is maintained by a combination of the tonic contraction of the cricopharyngeus muscle and the elastic properties of the gastrointestinal tract.3
Kaplan84 in 1957 and Sutherland85 in 1962 were among the first to advocate cricopharyngeal myotomy for treatment of dysphagia. Kaplan reported marked improvement in dysphagia and discontinuation of gastrostomy feeding in a patient with poliomyelitis, and Sutherland presented a series of eight patients with relief of dysphagia after myotomy for cricopharyngeal achalasia. In addition to primary muscle disorders of the cricopharyngeus muscle, indications have extended to include patients with Zenker's diverticulum, inclusion body myositis, oculopharyngeal muscular dystrophy, cerebrovascular accident, motor neuron disease, and a variety of neurologic disorders that result in disorders of pharyngeal pulsion.78, 86
Success rates of cricopharyngeal myotomy for treatment of oropharyngeal dysphagia have been unpredictable, ranging anywhere from complete failure to 100% success depending on the series.87 The mixed results may be attributed to the varied indications and current lack of understanding of the means by which many of the diseases in which cricopharyngeal myotomy is utilized affect deglutition. Myotomy for Zenker's diverticulum, for example, is consistently reported to have success rates in excess of 80%, whereas a review of 26 reports of myotomy including 419 patients for causes other than Zenker's diverticulum revealed a satisfactory outcome in only 61% of the cases.4, 81, 88, 89, 90
Refining the indications for surgery as well as careful patient selection may improve results while avoiding unnecessary procedures. Ross et al.,91 utilizing selection criteria that ensured patients had full use of voluntary oral musculature and a vigorous pharyngeal pressure wave, demonstrated the absence of upper esophageal sphincter relaxation during swallow, was able to predict with 90% accuracy those patients who would benefit from myotomy. Without absolute indications for cricopharyngeal myotomy, the vast majority of patients must be considered individually on a clinical basis.
External Approach to Cricopharyngeus Muscle Myotomy
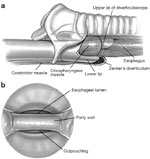
An oblique, transverse, or S-shaped cervical incision can be used for access. Most surgeons prefer the left side because the recurrent laryngeal nerve runs in a more vertical direction closer to the trachea. After careful dissection the sternocleidomastoid muscle and carotid sheath are retracted laterally. The laryngopharynx is then mobilized by blunt dissection to expose the cricopharyngeus muscle. An intraluminal esophageal Foley catheter or the cuff of a second endotracheal tube inserted into the esophagus can be inflated to facilitate visualization of the horizontal fibers of the cricopharyngeus muscles.92, 93
If indicated, a section of the muscle can be taken for biopsy before a posterior midline incision is made beginning approximately 1 cm superior to the start of the cricopharyngeus and extending inferiorly to include 1 cm of the circular esophageal fibers for a total of approximately 4 cm (Figure 14). It is important to include the striated muscle of the esophagus in the incision, as the upper esophagus has been shown to share the same pathology when the cricopharyngeus is affected.94 Intact esophageal mucosa should be visualized after myotomy. Any violation of the esophageal mucosa should be immediately repaired to prevent fistula or mediastinitis.
Figure 14: External approach to cricopharyngeal myotomy.
a: Incision of cricopharyngeus muscle. b: Posterior view of pharynx, esophagus, and trachea. Length of myotomy is indicated by dotted line. (Source: Kelly.93, with permission)
Endoscopic Approach to Cricopharyngeus Muscle Myotomy
Endoscopic myotomy of the cricopharyngeus is mainly a treatment for Zenker's diverticulum and is not widely used to address cricopharyngeal dysfunction or dysphagia of other causes.77 The endoscopic technique has traditionally been reserved for elderly patients with significant comorbidities because it has the perceived advantages of shorter operating time and thus less anesthesia risk as well as quicker postoperative recovery.
Endoscopic techniques for cricopharyngeal myotomy were available as early as 1917 but quickly fell into disfavor after mediastinitis emerged as a not infrequent complication.95 In 1960, with subsequent refinements in technique Dohlman and Mattsson96 helped the endoscopic approach remerged as viable alternative to the external approach.
A number of methods of endoscopic cricopharyngeal myotomy exist but all share a basic scheme. Visualization of the diverticulum is accomplished through an endoscope, and cautery, laser, or a stapling device is used to divide the common party wall between the outpouching and the esophagus (Figure 15).97, 98, 99 In addition to now incorporating the diverticulum into the native esophageal lumen, resection of the party wall is equivalent to a cricopharyngeal myotomy as the party wall is composed of the cricopharyngeus muscle.
Figure 15: Method of endoscopic cricopharyngeal myotomy.
a: Diverticuloscope advanced so upper lip is within esophagus and lower lip is within diverticulum. b:View through diverticuloscope. Cautery, laser, or a stapling device is used to divide the common party wall between the outpouching and the esophagus. (Source: Shapiro J, Van Overbeek J. Endoscopic laser cricopharyngeal myotomy. Oper Tech Otolaryngol–Head Neck Surg 1997;8(4):209-212 with permission.)
Scher and Richtsmeier98 reported the use of a bivalved Weerda laryngoscope utilizing video monitoring to straddle the party wall and subsequently divide and staple it with a gastrointestinal anastomosis stapler (Endo-GIA 30 stapler, US Surgical, Norwalk, CT). This modification addresses the concern of some surgeons about the unsutured incision through the cricopharyngeus that is required in other techniques of endoscopic myotomy.77
Complications
Postoperative complications are in general rare but include wound infection, hemorrhage, inadequate sectioning of the upper esophageal sphincter, transient unilateral vocal cord paralysis, recurrent laryngeal nerve damage, esophageal mucosal perforation, pneumomediastinum, and mediastinitis.93, 100 Based on the approach, injury to the recurrent laryngeal nerve would be more likely with an open incision whereas pneumomediastinum is more likely to occur with esophageal perforation during rigid esophagoscopy.
Surgical Access for Enteral Feeding
Although cricopharyngeal myotomy is intended to facilitate alimentation along the natural pharyngeal path, surgical enteral access bypasses the natural route with the goal of preventing aspiration and malnutrition. Enteral feeding is indicated in patients who have an intact, functional gastrointestinal tract but who are unable to process sufficient calories to meet metabolic demands. The enteral route is generally preferred over parenteral methods owing to preservation of gut integrity and function, decreased infectious complications, and the cost/benefit ratio.101, 102, 103. Surgical access for enteral feeding is considered when patients have a greater than 4-week projected need for supplemental alimentation. The most appropriate method for long-term enteral feeding is via a gastrostomy or occasionally a jejunostomy. Other techniques such as cervical esophagostomy are usually unnecessary in patients with dysphagia and protein energy malnutrition owing to oropharyngeal motor dysfunction. Currently, there are three methods of creating a gastrotomy: surgically via laparotomy or under either endoscopic or radiologic guidance.
Surgical Gastrostomy
Surgical gastrostomy was first described by Egeberg in 1837, but it was not until 1876 that Verneuil carried out the first successful gastrostomy.101, 104 In this era of minimally invasive surgery, open gastrostomy is rarely indicated for oropharyngeal dysphagia unless laparotomy is being carried out for other reasons.105 Nevertheless, open surgical gastrostomy was the first successful technique for long-term surgical enteral access and is the gold standard by which new techniques are measured.
After laparotomy, the Stamm method is commonly used to create the gastrostomy.106 A circular purse-string suture is placed in the body of the anterior wall of the stomach, and a second purse-string suture is placed outside the first circumferential suture. The feeding catheter is then brought through the abdominal wall and placed through a gastrostomy created in the center of the purse-string sutures. The purse-string sutures are tightened and secured, thereby inverting the gastric mucosa. Lembert sutures are used to secure the stomach to the abdominal wall.
Percutaneous Endoscopic Gastrostomy
Gauderer et al.107 reported the first percutaneous endoscopic gastrostomy (PEG) in 1980 in an attempt to reduce morbidity and mortality associated with gastrostomy in children. Owing to the relative ease in placement, cost savings, and ability to place under local anesthesia, PEG has become the preferred method in both children and adults.108, 109
Absolute contraindications to PEG placement include inability to bring the anterior gastric wall in apposition to the abdominal wall, pharyngeal or esophageal obstruction, total gastrectomy, peritonitis, uncorrectable coagulopathy, and patients unable to undergo conscious sedation, particularly those with severe respiratory disease. Absence of transillumination, and neoplastic, inflammatory, and infiltrative disease of the gastric and abdominal walls are relative contraindications.102, 108, 109
The most widely used technique of PEG is Gauderer's "pull" method. After an initial survey of the upper gastrointestinal tract, the stomach is insufflated with air and the tip of the endoscope is used to transilluminate the anterior abdominal wall (Figure 16a). Using the transilluminated skin as a guide, the stomach is punctured with a needle and a guidewire introduced over the needle and then snared under direct vision through the endoscope (Figure 16b,c). The guidewire and endoscope are then withdrawn through the mouth and the gastrostomy tube passed over the guidewire through the esophagus into the stomach with the tapered end passing out the anterior abdominal wall (Figure 16d,e).
Figure 16: Method of percutaneous endoscopic gastrostomy.
a: Transillumination of stomach to identify puncture site. b: Transabdominal insertion of needle into stomach under endoscopic visualization. c: Guidewire is passed into stomach and then grasped with snare. d: Guidewire is pulled caudally until it exits the mouth. e: Gastrostomy tubed passed over the guidewire until the tapered end exits the anterior abdominal wall. (Source: Kudsk KA, Jacobs DO. Nutrition. In: Surgery: Basic Science and Clinical Medicine. Norton JA, et al., eds. New York: Springer-Verlag, 2001(2) Part 7, Section 91:136., with permission)
The "push" variant utilizes the Seldinger technique and endoscopic visualization to pass the gastrotomy tube into the stomach through the abdominal wall instead of through the mouth. A prospective randomized trial has shown both techniques to have comparable results.110
With recurrent vomiting and tube feeding–related aspiration or severe gastroesophageal reflux the PEG can be converted to a PEG-jejunostomy by passing a jejunal feeding tube through the previously placed PEG.111 Though positioning of the tube distal to the ligament of Treitz should decrease feeding related aspiration, results have been colored by frequent retrograde tube migration into the stomach and kinking or obstruction. As a result, PEG-jejunostomy has not been convincingly shown to decrease enterorespiratory aspiration compared to conventional PEG.112, 113, 114
Percutaneous Radiologic Gastrostomy
In 1981, the year after Gauderer's paper describing PEG, Preshaw115 reported the first percutaneous gastrostomy placed under fluoroscopic guidance. Subsequently, several authors concurrently described fluoroscopically guided percutaneous radiologic gastrostomy (PRG) using a modified Seldinger technique.116, 117, 118
Both PEG and PRG are well-established, safe, and effective techniques for percutaneous gastrostomy.101 However, they differ on a number of points: PEG, unlike PRG, can be performed at the bedside and adds diagnostic capabilities. The rate of abdominal findings during PEG has been reported to be between 10% and 71%119. On the other hand, PRG negates the risks associated with esophagogastroduodenoscopy and can be used in situations were passage of an endoscope is ill advised or not possible.120
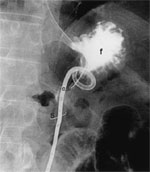
Percutaneous radiologic gastrostomy is performed in the radiology suite utilizing biplanar fluoroscopy. A nasogastric tube is inflated with air to displace the liver and colon as well as to get the anterior gastric wall close to the abdominal wall. The inflated stomach is punctured with a needle, which is then replaced over an angiography wire with a peel-off sheath with an introducer. One of a wide variety of gastrostomy catheters (Cope type vs. Foley catheters) is then inserted through the sheath after the introducer is removed.121Figure 17 shows a radiograph of a Cope loop gastrostomy tube in position within the stomach.
Figure 17: Radiograph of a Cope loop gastrostomy tube in position within the stomach.
A 12-French Cope loop gastrostomy tube (c) was placed after placement of a Cope suture anchor (s). Injection of contrast medium confirms the catheter loop in the fundus of the stomach (f). The nasogastric tube (N) has been introduced into the stomach for air insufflation. (Source: Texbook of Gastroenterology, Yamada T, et al, eds. 4th Ed. Phildelphia: Lippincott, Williams & Wilkins; 2003:3268, with permission)
Complications
Complications from surgical enteral access can be classified as either major or minor. Minor complications include peristomal infection, granulation tissue, minor bleeding, ileus, and tube clogging or malposition. Peritonitis requiring laparotomy, abscess, necrotizing fascitis, dislocation of the tube, bowel perforation, gastrocolic or colicocutaneous fistula, and aspiration (after the first week) are considered major complications. Peristomal infection is the most commonly minor complication in most series, whereas peritonitis remains the most frequent major complication.101, 122, 123 The exact categorization varies from report to report, which makes comparison among some studies difficult.
A review of the literature shows that the reported complication rates for surgical gastrostomy (1% to 43%), PEG (17% to 33%), and PRG (13% to 36%) varies widely.116, 124, 125, 126, 127. Procedure related mortality for PEG and PRG is routinely reported to be under 1%, whereas surgical gastrostomy in a large meta-analysis had a mortality rate of 2.5%.108, 112, 123, 128 Two recent series, however, reported no significant differences between the three techniques in terms of procedure-related complications and mortality.101, 105 Given these findings, the choice of technique should depend on the skill set of the operator as well as the individual necessities of the patient.
Conclusion
Surgical intervention for the varied causes of oropharyngeal motor disorders can be categorized as the treatment of chronic aspiration or dysphagia. Few disease state–specific treatments exist. Instead, surgical interventions aim to protect the airway, ameliorate dysphagia, or provide alimentation by either correcting anatomic problems or by bypassing affected steps in deglutition.
Treatment of chronic aspiration can be categorized by voice-sparing and voice-sacrificing techniques as well by reversibility. Proper treatment selection requires a thorough understanding of the etiology of the problem and consideration of the patient's overall prognosis and wishes.
Current surgical therapy for dysphagia of oropharyngeal motor dysfunction origin is mainly limited to cricopharyngeal myotomy and surgical access for enteral feeding. The variable results of cricopharyngeal myotomy indicate that more stringent selection criteria and a better understanding of the etiology of the dysphagia are needed. Refinements in percutaneous gastrostomy techniques have made gaining long-term enteral access a relatively easy and safe prospect. Nonetheless, the fact that a potentially life-prolonging procedure can be done safely doesn't necessitate its implementation in all patients. The overall 30-day mortality after PEG and PRG remains high, suggesting that selection criteria for long-term enteral access could also use some refinement.102, 112, 121, 123, 125 Even so, newer techniques such as skin level gastrostomies are expanding its appeal and allowing gastrostomy patients to be more active.127, 128
Surgical interventions are available to address the major symptoms of oropharyngeal motor disorders, but more work is needed to develop disease state–specific treatments that address the underlying deficit. The next generation of interventions should help restore sensory or motor function of the oral and pharyngeal area possibly through nerve or neuromuscular grafts.131, 132
Web Sites
The following Web sites provide further information:
1. Percutaneous dilational tracheostomy slideshow. URL: http://www.cookgroup.com/cook_critical_care/education/slides/index.html
2.Ciaglia Blue Rhino Percutaneous Tracheostomy Introducer Set Product Video. URL: http://www.cookgroup.com/cook_critical_care/education/video/index.html