Key Points
- Normal, safe, aspiration free swallowing requires the coordination of many of the neuromuscular structures within the head and neck, integrated with functioning anatomy and physiology.
- Normal, safe, aspiration free swallowing can be affected by:
- Loss of salivary flow
- Mucosal injury
- Muscular weakness
- Sensory deficits
- Connective tissue fibrosis
- Bony and soft tissue masses
- Respiratory disorders
- Neural pathway disorders
Introduction
Normal deglutition requires a complex and coordinated interaction of many head and neck structures. Abnormalities and disorders of oropharyngeal anatomy and physiology at any level will lead to some perceptible dysphagia. Normal human swallow is a combination of volitional events and involuntary reflexes.
This review discusses several of the diseases and disorders of the head and neck that can affect swallow function. The most clinically relevant method of organization is by disease category, allowing for a more global characterization of the dysphagia induced by each disorder. Other resources are provided for an in-depth understanding of the normal anatomy and physiologic events that occur during a normal swallow. Specifically, this review addresses anatomic abnormalities, including congenital, postsurgical, or tumor-related changes in oropharyngeal anatomy; neurologic and neuromotor abnormalities that interfere with both the reflexive and voluntary motor functions required for a normal oropharyngeal swallow; infectious diseases resulting in oropharyngeal dysphagia; iatrogenic, pharmacologic, and psychiatric causes of dysphagia; and gastroesophageal reflux disease and its probable role in oropharyngeal dysphagia (Table 1).
Anatomic Abnormalities Inducing Head and Neck Swallowing Disorders
Anatomic abnormalities in the head and neck resulting in dysphagia can be grouped into three categories: congenital abnormalities, traumatic injuries, and surgical insults. The primary congenital abnormalities associated with dysphagia are listed in Table 2. These disorders manifest themselves in all cases during infancy. Traumatic abnormalities are caused by direct injury, surgical scaring, radiation therapy, thermal injury, or caustic ingestion. The complex anatomic problems associated with head and neck cancer and its treatments will be discussed separately.
Congenital Abnormalities
Cleft Lip and Palate
Cleft lip with resulting oral incompetence and cleft palate with resulting velopharyngeal incompetence can lead to dysphagia. These craniofacial abnormalities may occur independently or in combination with other anatomic abnormalities and can be associated with other syndromes. A cleft palate can preclude an infant from creating appropriate intraoral negative pressure during suckling. The resulting dysphagia may require mechanical assistance in the form of a modified nipple, or even a temporary tube feeding. Early surgical intervention is now commonly the case, with the repair of both the cleft lip and palate performed often within the first year of life.1, 2
Choanal atresia and laryngomalacia can also lead to swallowing disorders in newborns. In both cases, the swallowing disorders are the result of respiratory difficulty during feeding. Choanal atresia, partial or complete, disrupts breathing during feeding as the child cannot move air through the nose and nasopharynx. Laryngomalacia is primarily a respiratory disorder, but when severe it can disrupt feeding owing to the work of breathing and an association with gastroesophageal reflux.
Laryngeal Clefts and Tracheoesophageal Fistula
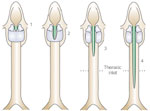
Laryngeal clefts are categorized by the extent of clefting, ranging from type 1, which involves the interarytenoid space, to type 4, which extends down into and beyond the thoracic inlet, involving the trachea and upper esophagus (Figure 1).3 Large laryngeal clefts are incompatible with the normal swallow as aspiration occurs with all swallowed and/or refluxed materials. Surgical closure is required prior to oral alimentation. On the other hand, a tracheoesophageal fistula may manifest as a subtle, difficult to diagnose, small fistulous tract presenting with recurrent pneumonias in the infant.4Other associated abnormalities may include tracheal stenosis and esophageal atresia.4
Figure 1: Laryngeal clefts classification.
Type 1: supraglottic interarytenoid cleft. Type 2: partial cricoid cleft. Type 3: total cricoid cleft. Type 4: laryngoesophageal cleft. (Source: Benjamin and Inglis,3 with permission from Annals Publishing Co.)
Micrognathia (Mandibular Hypoplasia)
Isolated micrognathia and micrognathia associated with disorders such as a Pierre Robin sequence (micrognathia, glossoptosis, and cleft palate) result in dysphagia secondary to posterior tongue displacement. This anatomic abnormality can lead to poor oropharyngeal intake and difficulties with upper airway maintenance. Infants often require intervention. Nasogastric tube feeding or even gastrostomy tube feeding may be needed to provide the child with the necessary nutrition to sustain life. Early airway intervention with mandibular distraction or tracheostomy has been described to achieve a safe airway.
Vascular Abnormalities
Vascular abnormalities of the great vessels have been described, leading to primarily esophageal obstruction. The most commonly identified include compressive vascular rings secondary to right-sided aortic arch or double aortic arch, or compression secondary to aberrant right subclavian artery (dysphagia lusoria). These disorders are easily diagnosed with contrast radiology. Severe symptoms require surgical intervention with resection of the aberrant right subclavian artery and vascular rerouting.5
Traumatic and Postsurgical
The dynamic nature of swallowing requires mobility of structures. Laryngotracheal elevation and pharyngeal contracture occur during a normal swallow. Limitation or impairment of mobility leads to dysphagia. The presence of a tracheotomy, scarring, fibrosis, or mucosal trauma from ingestion of caustic materials can impede normal movement and result in dysphagia. A later section covers cricopharyngeal dysfunction and Zenker's diverticulum.
Tracheotomy
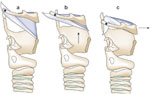
The association between tracheotomy and dysphagia has been somewhat controversial. A three-tiered process leads to a safe swallow and prevention of aspiration. Laryngeal closure with laryngeal elevation under the overlying hyoid and downfolding of the epiglottis over the top of closed vocal folds protects the airway during swallowing (Figure 2).6 Tethering of the trachea to the skin by a tracheotomy can prevent this final epiglottic downfolding, leading to bolus transport toward a closed glottis with secondary aspiration upon opening.7 Tracheotomies may not only tether the larynx in an inferior position, but also interfere with the normal respiratory events that surround swallow, including the creation of subglottic pressure and interference with the patient's ability to produce an adequate cough. In canine studies, the presence of an open tracheotomy affects the sensory, motor, and reflex activities of the larynx.8 However, the great majority of patients with a tracheotomy owing to airway disorders such as glottic stenosis, subglottic stenosis, or sleep apnea are able to compensate adequately and produce an essentially normal swallow. In the acute patient setting, no causal relationship was found between tracheotomy and aspiration.9
Figure 2: Mechanism of epiglottic downfolding.
a: Pre-swallow position for larynx and hyoid. b: First superior movement of laryngeal elevation below hyoid. c: Second movement of larynx anterior with epiglottic tip inferior deflection. (Source: Van Daele et al.,6 with permission from Blackwell Publishing.)
Intrapharyngeal Scars
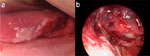
Intrapharyngeal scarring produced by lye ingestions or thermal injuries can result in significant obstructive dysphagia (Figure 3). In severe cases superficial scarring at the level of the pyriform sinus and cervical esophagus leads to complete inlet obstruction. The treatment for this type of scar formation is in most cases dilatation of the scarred region. In severe cases, the obstructed level must be bypassed. Techniques are available for complete pharyngeal and esophageal bypass procedures, which allow patients to resume a nearly normal diet, as long as their pharyngeal musculature is intact and they are able to produce adequate bolus propulsion pressures (Figure 4).10
Figure 3: Pharyngeal lye injection.
Pharyngeal Lye injection. a: Lateral tongue with mucosal injury. b: Hypopharynx with severe mucosal injury.
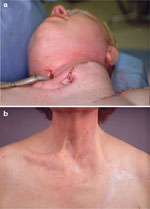
Figure 4: Radial artery forearm free flap reconstruction of cervical esophagus.
a: Initial stage of reconstruction with radial forearm free flap pharyngeal reconstruction open onto anterior chest wall. b: After colon interposition and attachment of reconstructed pharynx.
Cervical Spine Approaches and Osteophytes
Dysphagia associated with cervical osteophytes has been reported by many individuals. The association is primarily with large lesions below the level of C3. It is not completely understood whether the dysphagia is owing to the obstruction of the cervical esophagus from the mass of the osteophyte or in part owing to the inflammatory events that occur around osteophyte formation. On occasion, the lateral barium swallow will illustrate bolus obstruction above the level of the osteophyte. Treatment initially consists of diet manipulation and on occasion proceeds to surgical intervention by means of removal of the osteophyte. Complete resolution of the dysphagia after surgical excision, however, is not always seen.11, 12 The surgical approach to the anterior cervical spine can lead to cranial nerve injury and scar formation that may interfere with swallow function to a greater degree than does the anterior osteophyte. The risk versus the benefit of surgery must be diligently evaluated. The patient's dysphagia should be scrutinized for other causes, such as sensory or motor deficits prior to surgical intervention.
Cricopharyngeal Dysfunction and Zenker's Diverticulum
Relaxation of the cricopharyngeus is an essential component of a normal pharyngeal swallow event. The failure of complete relaxation can lead to prolongation of bolus transit times and is associated with elevated intrapharyngeal pressures. When the bolus fails to completely reach the esophagus, the residual material remains within the piriform recess, at risk for post-swallow aspiration. A variety of disorders may lead to cricopharyngeal dysfunction, including primary achalasia, polymyositis/dermatomyositis, neuromuscular discoordination poststroke, or peripheral nerve injury and aging.13, 14, 15, 16 Treating the cricopharyngeal problem can involve dilatation, myotomy, or the use of intramuscular botulinum toxin.13 When the cricopharyngeal dysfunction is a part of global pharyngeal weakness, as is the case with an amyotrophic lateral sclerosis (ALS) patient, interventions at the level of the cricopharyngeus fail to compensate for the primary bolus propulsion loss.
Zenker's diverticulum, a posterior hypopharyngeal outpouching, is thought to be primarily the result of cricopharyngeal abnormalities coupled with a natural weakness inferior to the pharyngeal constrictor muscles and secondarily elevated swallow pressures. Zenker's diverticulum is a disorder of an aged population. The average age of diagnosis is around 70 years, and it is rarely seen prior to age 50.17 Studies of swallowing pressure have demonstrated that with age bolus transit times increase, upper esophageal pressure drop is delayed in time, and the minimum upper esophageal opening pressure increases to a significant positive value.18 These changes with age, coupled with a natural predisposition for posterior pharyngeal outpouching, are the likely mechanisms for Zenker's diverticulum formation.19
The surgical treatment of Zenker's diverticulum requires some form of cricopharyngeal myotomy, with or without the removal of the diverticulum itself. The current trend is to treat it transorally using a CO2 laser or stapling techniques (Figure 5).19 The results from either technique are reported to be quite good, with the risks of endoscopic treatment possibly lower than with open techniques.20, 21
Figure 5: Transoral laser assisted cricopharyngeal myotomy.
a: Cricopharyngeal bar visible anterior to Zenker's diverticulum (arrow). b: After laser cricopharyngeal myotomy, with diverticulum open into esophagus.
Head and Neck Tumors and Malignancy
The initial dysphagia associated with head and neck malignancy is attributed to the combination of disrupted normal anatomy secondary to mass effect, nerve involvement, soft tissue tethering, or tumor induced pain. The majority of head and neck malignancies are mucosally based and are squamous cell carcinomas. These lesions can be exophytic or infiltrative in nature, and often metastasize to cervical lymph nodes requiring treatment of not only the primary location but also draining lymphatics. Persistent swallowing deficits after treatment are related to the magnitude of surgical intervention or soft tissue and tumor response to nonsurgical therapies such as radiation and chemotherapy.
Tumors of the oral cavity, including the anterior tongue, buccal mucosa, alveolar ridge, and anterior palate are better compensated for than tumors of the oropharynx, which include the tongue base, tonsillar fossas, and posterior pharyngeal wall.22 The surgical treatment of oral cavity carcinoma can often restore structure and sometimes function. The portion of the swallow that occurs in the oral cavity is primarily voluntary and is associated mostly with bolus manipulation and mastication (Figure 6). Recovery of the involuntary or reflexive portion of the oropharyngeal swallow is much more difficult, as sensory and motor deficits lead to significant limitations in bolus pressure and propulsion.
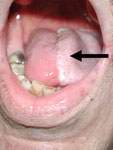
Figure 6: Tongue reconstruction with radial forearm free flap.
Native tongue on right and free flap left hemitongue (arrow).
Small defects in the tongue base (less than one third) can often be closed primarily or allowed to heal by secondary intention. The majority of these patients do not manifest significant dysphagia. However, defects beyond one third of the tongue base often require closure utilizing transfer of tissue from distant sites, either with regional flaps or free tissue transfer techniques (Figure 7). Independent of the reconstruction technique used, the most important factor with regard to swallowing rehabilitation is the magnitude of tongue base resection.23, 24 Advocates of laser surgeries emphasize the advantage of preserving function by eliminating the trauma from the surgical approaches through the neck with the hope of retaining sensation, as well as preventing tethering from the additional scar. A 2003 report by Steiner et al.25 reports that 92% of patients were able to return to normal diets after transoral laser excision of tongue base tumors.
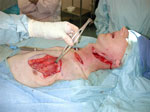
Figure 7: Pharyngeal closure with pectoralis major skin and muscle flap.
Rotation of pectoralis major skin and muscle flap into neck to repair pharyngeal defect.
Surgical resection can have devastating effects on swallowing. For example, total glossectomy patients always have dysphagia, and if resection extends beyond the tongue to laryngeal or pharyngeal structures these patients may never be functional oral eaters. However, if less than one half of the oral tongue is removed followed by primary closure, most patients are able to regain nearly normal oral eating.23, 26 Swallowing is more significantly affected if surgical reconstruction involves placement of a flap. This replacement tissue is adynamic and at least initially insensate. Patients treated primarily with surgical therapy often demonstrate improved function over time as healing progresses, the scar tissue softens, sensory recovery proceeds, and patients adapt compensatory strategies.
Nonsurgical therapy for head and neck cancer patients has become quite common in 2005. The combination of chemotherapy and radiation produces a high rate of local/regional tumor control, and in some cases appears to delay the onset of metastatic disease.27, 28, 29 Unfortunately, the elimination of the cancer can leave devastating side effects, including the inability to eat and swallow normally. Organ preservation, often assumed to be the preferred treatment, has now been shown to leave functional deficits, which prolong and magnify the dysphagia.30 The incidence of swallowing problems in these treatment groups is extremely high and may even worsen with time.31, 32 The treatments can lead to significant soft tissue fibrosis, mucosal scaring, and xerostomia. Long-term sequelae of radiotherapy can include loss of energy, pain, xerostomia, impaired taste, impaired smell, trismus, tooth decay, tissue stiffness, edema, and impairments in speech, voice, and swallowing. Conventional therapies for dysphagia are of limited benefit, and many patients are left with severe, lifelong dysphagia.33 This problem has a major impact on the quality of life of these cancer survivors and often affects nutrition, hydration, and pulmonary health.34, 35 One report found that 12 months after treatment, only 56% of all head and neck cancer patients were eating a normal diet.36
In a large randomized clinical trial of radiation therapy vs. chemotherapy and radiation therapy (chemorads), patients were followed for an extended time posttreatment.37The combined treatment significantly improved tumor control and disease-free survival but was associated with a substantial increase in adverse effects. Using the common toxicity criteria of the National Cancer Institute and the Radiation Therapy Oncology Group (RTOG) criteria, initial problems were identified in 34% of the radiation group and 77% of the chemorads group.37, 38Problems with the pharynx and esophagus (swallow) were reported as common. In the late stages, greater than 90 days, one in five patients reported problems with swallowing, followed by salivary and laryngeal problems.37
This reported incidence is likely an underestimate of the true extent of the problem. It appears that head and neck cancer patients' self-report of swallowing is better than actual swallow performance as visualized fluoroscopically or endoscopically.31, 39, 40 Using imaging tools, several reports have found that virtually all patients had swallowing abnormalities in the late stages after radiation therapy. Wu et al.40 evaluated patients treated with radiation for nasopharyngeal cancer and found that over 90% have a definable abnormality on endoscopic swallowing examination.
The need for a feeding tube is a good indication of a significant swallowing problem. One retrospective study found 32% of radiation patients and 69% of chemorads patients received a feeding tube during treatment.41 Staar et al42 reported that 25% of radiation patients and 51% of chemorads patients still needed a feeding tube 2 years after treatment. This high incidence may be partly an effect of the philosophy of the treating physician. A significantly lower incidence (9%) was reported by List and Bilir43 in a summary article on toxicity of chemorads in the head and neck population.
The nature of the dysphagia after chemorads has been described in various articles. One study compared fluoroscopic findings to observed structural changes with computed tomography studies in patients post-chemorads.35 The authors concluded that pharyngeal constrictors and laryngeal adductors showed structural changes that could be attributed to the chemorads. The fluoroscopy studies showed all movements comprising the swallow were impaired, including tongue-base retraction and hyolaryngeal excursion. It seems that the entire swallow mechanism is affected. Supporting this concept, Lazarus44 found that the location of tumor had very little effect on the nature of the swallowing problem post–radiation therapy; all patients post-chemorads had a weak swallow, and some of them aspirated or allowed food or liquid into the airway. These results are similar to those of Wu et al.'s40 endoscopic study in which greater than 90% of the patients post-chemorads aspirated or penetrated.
In summary, dysphagia associated with head and neck malignancy is extremely common and may remain the primary residual side effect of treatment even when the tumor is controlled.
Neurogenic and Neuromuscular Dysphagia
Cranial Nerves
Neural control, including both sensory input and motor function, plays an integral role in swallowing. Sensory stimuli provide information with regard to bolus location and aspiration prevention, whereas neuromotor function assists in pressure formation that is required for bolus propulsion during all phases of swallowing. Either sensory or motor neural deficits can lead to oropharyngeal dysphagia. Isolated cranial nerve (CN) deficits can include the facial nerve (CN VII), glossopharyngeal nerve (CN IX), vagus nerve (CN X), and hypoglossal nerve (CN XII). Cranial nerve conduction problems can result from a number of causes including direct trauma to nerves, tumor infiltration or compression, or postviral illness. Dysfunction of the facial nerve leads to poor oral commissure control and limitations of bolus manipulation within the oral cavity during mastication. Hypoglossal nerve deficits affect bolus manipulation within the oral cavity and interfere with bolus delivery into the oropharynx. The glossopharyngeal nerve has both a sensory and motor division. The areas innervated include the tongue base and lateral pharyngeal walls, which are important in triggering the reflexive portion of the pharyngeal swallow. This nerve also contributes to the pharyngeal plexus, which controls the motion of the constrictor muscles utilized in bolus propulsion and clearance. Isolated glossopharyngeal nerve weakness is rare; in most cases it is found in combination with vagal nerve or polynerve dysfunction (Figure 8). The vagus nerve also has both motor and sensory components. The sensory component is primarily represented by the superior laryngeal nerve, which provides sensation across the epiglottis, false vocal folds, and portions of the pyriform sinus. Sensory deficits in these areas lead to poor initiation of swallow reflex, primarily with liquid boluses, which often pool in the pyriform sinuses prior to swallowing. The motor division of the vagus is responsible for true vocal cord motion and contributes to the pharyngeal plexus. The complex clinical problems created by vagal nerve injury are discussed below.
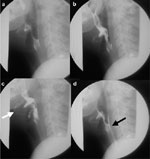
Figure 8: Barium swallow study after cranial nerve injury (IX, X, and XII) with asymmetric hyoid elevation.
a: Pre-swallow. b: Bolus delivery to hypopharynx. c: Laryngopharyngeal elevation (white arrow). d: Post-swallow with residual barium in piriform sinus (black arrow).
Vagal Nerve Injury and Vocal Cord Paralysis
The primary motor division of the vagus nerve is the recurrent laryngeal nerve, which is responsible for the motion of the intrinsic laryngeal musculature. Its primary importance in swallowing is its ability to produce vocal cord adduction during the bolus passage, and to allow appropriate glottic closure during the cough reflex. Injury to the recurrent nerve leads to ipsilateral vocal cord paralysis. Symptoms often include dysphagia, primarily with liquid, weak voice, and poor cough. Of more significance is vagal nerve injury at or near the skull base. This adds a component of pharyngeal motor weakness and a laryngopharyngeal sensory deficit significantly compounding the aspiration risk. Tabaee et al.45 in 2005 reported a 35% aspiration incidence in their patients with unilateral paralysis. The same group of patients had a documented sensory deficit 80% of the time and an absent laryngeal adductor reflex in 34% of the group. High vagal nerve injuries often include injury to adjacent nerves at the skull base, including the glossopharyngeal and hypoglossal nerves. The combined insult produces unilateral pharyngeal sensory and motor deficits, which involve all the muscles used in bolus transfer. This produces severe and frequently unrecoverable dysphagia in most patients, and compensatory strategies are rarely successful.
Surgical interventions to improve glottic closure are well described.46, 47 Vocal cord injection procedures restore bulk to the atrophic and lateralized vocalis muscle. Injection materials include collagen, fat, Teflon, and others.48, 49 Open laryngoplasty procedures, which are completed through small neck incisions also allow repositioning of the vocal fold into the normal phonatory or cough position (Figure 9). These procedures may be more precise and permanent when compared to injection techniques. Recovery of sensory deficits induced by high vagal nerve injuries, however, cannot be surgically improved and in many cases require significant time for recovery or compensation.
Figure 9: Open laryngoplasty to medialize a paralyzed vocal cord.
Gore-Tex thyroplasty material is placed into paraglottic space from inferior to thyroid cartilage (arrow at inferior edge of cartilage).
Stroke
Central nervous system injury induced by hemorrhagic or ischemic stroke, both hemispheric and brainstem insults, can lead to dysphagia. At initial assessment, approximately 50% of stroke patients are at risk for aspiration. Over time, many of these patients improve such that by one week only one quarter of the patients remained at risk, and by 6 months less than 10%.50 Stroke and the associated dysphagia are complex issues are covered in detail in other reviews.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a degenerative motor neuron disorder of adults. This condition commonly manifests itself in its early stages with dysarthria and dysphagia. These patients classically show progressive debilitation. It most commonly presents in middle or late life and the etiology remains unknown. Patients usually present with limb weakness or with bulbar symptoms. In most patients ALS is a rapidly progressing disease, with death caused by respiratory failure. Treatment is primarily supportive. Pharyngeal weakness significantly affects bolus transport. Aspiration becomes more common as the disease progresses and the placement of a feeding tube becomes essential for nutritional support.17, 51 Treatment of respiratory weakness can require tracheotomy and mechanical ventilation. Weak or absent cough can lead to life-threatening pneumonia.
Myasthenia Gravis
Neuromuscular dysfunctions can also occur at the level of the neuromuscular junction; such is the case in myasthenia gravis. This is an autoimmune disorder in which antibodies that interact at the neuromuscular end plate are produced. This produces progressive weakness with repetitive muscle use. The most common presentations in the head and neck are ptosis, diplopia, dysarthria, and dysphagia. The diagnosis can be made by the detection of acetylcholine receptor antibodies within the blood, or the utilization of a Tensilon challenge test. Tensilon, a short-acting acetylcholinase potentiates the activity of acetylcholine at the motor unit endplate, temporarily alleviating symptomatology. Treatment usually consists of medical management with acetylcholinesterase (AChE) inhibitors, plasma exchange for the elimination of autoantibodies, immunosuppression, and occasionally surgical intervention by means of a thymectomy.52
Multiple additional neuromuscular disorders are associated with dysphagia. Multiple sclerosis, producing demyelination of the brain and spinal cord, is characterized by periods of remission and exacerbation. In its later phases it is commonly associated with dysphagia.51, 53Patients with Parkinson's disease, Huntington's chorea, and cerebral palsy are also frequently troubled with swallowing problems.
Autoimmune, Thyroid and Salivary Disorders
A variety of disorders that have major systemic manifestations, such as diabetes, may also contribute to systems of dysphagia. Many of these maladies could be classified as autoimmune disorders. Included in this group would be Sjögren's syndrome, myasthenia gravis, lupus, inclusion body myositis, and many thyroid disorders. Several of these topics have been discussed in other sections. In addition there are many nonautoimmune thyroid diseases that may lead to dysphagia.
Salivary Disorders
The salivary glands are essential for normal bolus formation during the oral phase of swallow, adding moisture and lubrication to the chewed material prior to the initiation of the pharyngeal phase of swallow. Primary dysphagia associated with the salivary glands is caused by disorders that interrupt normal salivary flow. Such conditions include Sjögren's syndrome, in which autoantibodies significantly diminish the salivary flow, leading to dry oral cavity and secondary changes in oral transport with prolongation of this phase of swallow.54, 55 The therapy for this disorder is essentially symptomatic with supplemental hydration utilized during eating.
The other primary cause of salivary dysfunction is radiation therapy. Xerostomia is seen in the head and neck region after head and neck malignancies are treated with external beam radiation therapy that encompasses the parotid and submandibular gland areas. Attempts are made to spare salivary glands when possible during radiation treatments; however, the nature of an oropharyngeal malignancy in almost all cases requires a significant portion of the salivary glands to be incorporated within the radiation field. Postradiation xerostomia is a lifelong problem requiring patients to utilize supplemental hydration with nearly all bolus consistencies. Although artificial salivas have been created, most patients find little utility in these products, and in almost all cases are required to carry water with them at all times. Xerostomia is likely the most common long-term complaint of a postradiation therapy patient.
Thyroid Diseases
Thyroid diseases are also occasionally associated with dysphagia. Thyroid enlargement associated with goiter can lead to some compressive symptoms owing to the thyroid's being adjacent to the esophagus. This situation is most likely to occur in patients who have substernal extensions of their goiter in which the goiterous mass occupies the narrowed space in the thoracic inlet. However, it is rare that patients develop severe dysphagia owing to compressive symptoms only.56
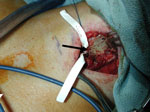
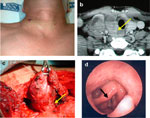
More concerning are thyroid malignancies. Although the majority of thyroid malignancies do not directly lead to dysphagia, it is not uncommon that extracapsular extension of thyroid tumors involves the tracheoesophageal junction and thus the course of the recurrent laryngeal nerve, leading to vocal cord paralysis and its secondary dysphagia consequence (Figure 10). The highly malignant forms of thyroid cancer such as the anaplastic variety directly invade surrounding structures. These patients may present with rapid onset of dysphagia as the tumors invade the cervical esophagus and surrounding neuromuscular structures.
Figure 10: Invasive thyroid cancer.
a: Neck mass. b: Computed tomography image (arrow at recurrent nerve). c: Surgical image (arrow at recurrent nerve). d: Clinical photo of larynx showing cord paralysis post treatment (black arrow).
Surgical intervention for thyroid disease is perhaps one of the most common causes of postoperative dysphagia secondary to vocal cord paralysis. Recurrent laryngeal nerve injury during thyroid surgery is estimated at between 1% and 10%, depending on the nature of the thyroid disease, benign or malignant, and the experience of the thyroid surgeon.57
Infectious Disorders as a Cause of Oropharyngeal Dysphagia
Several infectious disorders can lead to short-term dysphagia secondary to mucosal inflammation and swelling (Table 3).
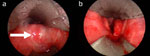
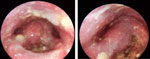
One of the more common inflammatory causes of dysphagia is candidiasis of the oral mucosa (Figure 11). Candidiasis occurs in the presence of mucosal inflammation owing to other initial irritants, such as radiation therapy. However, it is also seen in patients who have undergone immunocompromising treatments such as chemotherapy for hematologic malignancies. It is also not uncommonly seen after using antibiotics that disrupted the normal oral flora (which are naturally protective against yeast overgrowth). The common bacterial infections of the oropharynx, such as streptococcal pharyngitis, can lead to inflammatory changes of the mucosal surfaces with secondary pain and dysphagia. Similarly, abscess formation in the perioral structures such as the tonsils, floor of mouth, buccal space, and retropharynx can also lead to inflammatory changes, edema, and pain associated with severe dysphagia.
Figure 11: Laryngopharyngeal candidiasis with characteristic plaques.
Endoscopic views of larynx and hypopharynx.
Viral etiologies of dysphagia include herpes zoster, which may involve the distribution of any of the sensory branches of the vagus nerve, the glossopharyngeal nerve, the trigeminal nerve, or the lingual nerve. The primary manifestation occurs with vesicular eruptions along the mucosal surfaces innervated by the involved cranial nerve. These are associated with severe local pain and occasionally motor deficits.
Although rare today, other local infectious processes can manifest themselves in the oropharynx, including tuberculosis, diphtheria, and even gonorrhea. These produce local inflammatory changes that are not dissimilar to the more common infectious agents and are diagnosed only by appropriate culture techniques. Systemic infectious diseases can also manifest themselves with episodes of oropharyngeal dysphagia. The human immunodeficiency virus in its early stages can produce secondary Candida albicans infections and can be associated with major aphthous ulcers. Lyme disease, a systemic illness resulting from infection with the spirochete Borrelia burgdorferi, can occasionally manifest with problems such as facial nerve paralysis, pharyngeal pain, and associated dysphagia. The diagnosis is made on clinical grounds and by appropriate blood studies testing for the antibodies to Borrelia.58 Tetanus is an uncommon disease process induced by the gram-positive rod Clostridium tetani. This infectious process presents with muscle rigidity, often seen in the jaw and pharyngeal musculature. Its diagnosis is made by the clinical manifestation and a history of a prior wound, which may have acted as a nidus. It is not uncommon for tetanus to occur without the obvious wound history. The treatment for the most part is supportive but can occasionally include tetanus antiserum and/or the utilization of diazepam.
Pharmacologic Effects
Medication side effects may be one of the most common but unrecognized causes of oropharyngeal dysphagia (Table 4). Antibiotics are common causes of temporary mucositis with changes in protective normal oral bacteria. This condition is usually self-limited, resolving when the medication is stopped. However, secondary yeast infections can lead to severe mucositis requiring directed treatment and analgesics.
Many medications have anticholinergic side effects, which can include mucosal dryness, problems with bolus manipulation, chewing and swallowing, denture misfit, mucosal injury, dental decay, decreased thirst reflex, and weight loss59 (Table 4). Antipsychotic medication can affect swallowing via multiple pathways, such as through its anticholinergic effects at the levels of the salivary glands and the esophagus, and more significantly with the production of tardive dyskinesia causing lingual and upper esophageal hyperkinesias.60 Antihypertensives can affect swallowing by acting as anticholinergics, and as systemic drying agents (furosemide, thiazide) secondary to diuresis and dehydration. An additional class of antihypertensives, the angiotensin-converting enzyme (ACE) inhibitors, has been linked to spontaneous oral, pharyngeal, and laryngeal angioneurotic edema.
A unique medication, botulinum toxin type A, is used in many head and neck neuromuscular disorders such as spasmodic dysphonia and torticollis. It permanently blocks neuromotor end-plate function and leads to temporary muscle paralysis, which recovers only after nerve sprouting and growth of new end plates. Dysphagia is caused by the migration of the agent into the nearby muscles required for swallowing and airway protection.
Laryngopharyngeal Reflux
Laryngopharyngeal reflux is discussed in detail in other reviews of this online publication; however, it is reasonable to recognize that it is currently one of the most commonly diagnosed problems in patients reporting abnormal swallowing. In a study of 220 patients with unevaluated clinical symptoms of dysphagia, it was found that 18% of patients had reflux disease as the primary cause.61 The symptoms of laryngopharyngeal reflux are diverse and overlap with those of many other head and neck disorders. The relationship of reflux disease with swallowing complaints is weak. Only one in 10 patients with documented reflux has a swallowing related symptom.62 When patients present with vague symptoms commonly associated with reflux disease such as mild voice change, lump in the throat, mild pharyngeal discomfort, and dysphagia, it is of primary importance that other causes of these symptoms are kept in mind to be certain that life-threatening hypopharyngeal cancers are not missed.
Psychogenic Dysphagia
Psychogenic causes of dysphagia are rare, but real; however, like reflux-related dysphagia, it is important to rule out physiologic causes prior to focusing solely on the psychogenic. The role of psychological factors in the etiology of swallowing disorders remains controversial. Four types of functional dysphagia are recognized: (1) developmental, which is reported in children who are not taught to eat solid foods early in life; (2) conditioned dysphagia, in which a negative stimulus is paired with swallow leading to avoidance; (3) primary conversion reaction, in which a swallowing problem develops following an associated external psychic conflict; and (4) secondary conversion reaction, in which a dysphagia symptom can be linked to a prior traumatic swallowing event, such as a choking episode.63
Conclusion
During a normal swallow there is a complex coordination of nearly all oral, pharyngeal and laryngeal muscles, and sensory surfaces in these regions. When a disease or disorder affects any of these sites or structures, the orchestration breaks down, resulting in dysphagia. The magnitude of the swallowing problem depends on the extent of insult and the capacity of the patient to compensate. When both motor and sensory functions are affected, the problems are also significantly exaggerated. Although sometimes treatment options are limited, a proper diagnosis can guide therapy and assist the patient in understanding the etiology of the problem. It can also assist in the prevention of malnutrition and possible life-threatening pneumonia.