Key Points
- Electromyography (EMG) is the recording of the electrical phenomena that determine muscle health, muscle excitation and contraction. It is particularly useful in the study of the striated muscles.
- EMG is very useful in clinical diagnosis, understanding of physiology and pathophysiology, and treatment of oropharyngeal dysphagia
- EMG studies provide precise timing of activity of muscles and can be used even for very small muscles in vivo. EMG studies have provided very important information on the sequence of activation of muscles involved in oropharyngeal phase of swallowing
- Diagnostic electromyography (EMG) can determine a site of pathology along the lower motor neuron, and it can be used in the differential diagnosis of various neuromuscular diseases.
Introduction
In clinical medicine, electromyography is often looked upon as simply one of a variety of electrodiagnostic examinations that are performed in an effort to determine the presence and site of neuromuscular abnormalities. In fact, Stedman's Medical Dictionary1 defines electromyography (EMG) as "The recording of electrical activity generated in muscle for diagnostic purposes...." Because of the semantic implications associated with the word diagnostic, this definition has restrictive boundaries.
Although electrodiagnosis is likely the most common application of EMG, it is used in both clinical and bench research, with both humans and animals, to study the contribution of healthy nerves and muscles to the execution of voluntary and involuntary physiologic events. Additionally, it is used by a variety of disciplines for purposes of biofeedback.2, 3
Therefore, a broader definition in which EMG is defined as the recording of the electrical phenomena that are associated with the first stage in the sequence of events that link muscle excitation with muscle contraction4 may be more appropriate. For our purposes, this broader definition covers a range of applications, particularly as they relate to efforts to better understand healthy as well as diseased muscle function and the effects of medical and behavioral treatment techniques associated with normal and disordered swallowing.
Principles of Electromyography
Electromyography is the only technique that can directly display activity from a specific muscle. The functional unit of muscle contraction is the motor unit. The motor unit is composed of a single motor neuron with the cell body and dendrites that reside in either the brainstem or the spinal cord, the axon traveling from the motor neuron, the myoneural junction, and the muscle fibers innervated by that motor neuron.
At rest, a muscle fiber maintains a steady potential across the membrane. When an impulse travels along a nerve and arrives at the myoneural junction, acetylcholine is emitted from the motor end plate. This results in depolarization of the muscle fiber and a subsequent muscle contraction. The depolarization generates an electromagnetic field and the action potential is measured as a voltage. It is important not to confuse muscle contraction with muscle shortening. If a muscle antagonist is also contracting, the degree of antagonistic contraction limits the ability of a contracting muscle to shorten.
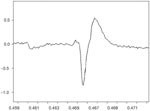
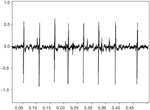
The motor unit action potential is the spatiotemporal summation of the individual muscle action potentials for all the fibers in the vicinity of a given electrode or electrode pair. Consequently, the EMG signal is the summation of the motor unit action potentials within the pickup field of a particular electrode or electrode pair. Figure 1 is expanded in the time domain in order to display a single motor unit action potential (MUAP) from the medial thyroarytenoid muscle of a healthy adult larynx. Figure 2 shows a train (MUAPT) of single MUAPs from that muscle with time more compressed.
Figure 1: Single motor unit action potential (MUAP).
This MUAP is from the medial thyroarytenoid muscle of a healthy adult larynx. The time domain is expanded.
Figure 2: Train (MUAPT) of single MUAPs from the thyroarytenoid muscle displayed in Figure 1.
The time domain is compressed more than in Figure 1.
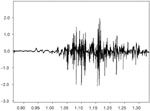
Any portion of the muscle may contain fibers belonging to as many as 20 to 50 motor units and a single motor neuron can innervate as few as three or as many as 2000 muscle fibers.5 Muscles controlling fine movements, such as those of the eye, human tensor tympani, larynx and pharynx,5 have smaller numbers of muscle fibers per motor unit, whereas those controlling gross movements, such as the gastrocnemius m., have a large number of fibers per motor unit. Usually the muscle fibers of different motor units are intermingled throughout a muscle, in which case, the pickup field of an electrode will include more than one motor unit. Figure 3 shows the firing pattern from bipolar hooked wire electrodes recording a collection of multiple MUAPs from the medial thyroarytenoid muscle.
Thorough explanation of the principles of EMG as are presented in classic textbooks, such as those by Lahoda et al.6 and by Basmajian and DeLuca,5 and muscle physiology has been well described by Aidley.4 Computer ability was just burgeoning at the time of these publications; thus references to digital recording and computer analysis are minimal or absent. Although strip chart recorders, magnetic tape recordings, and hand-held calculators have been replaced by analogue-to-digital converters and computer recording and analysis programs, the basic instrumentation required for data acquisition has not changed dramatically over the decades. Other readings to assist in understanding electromyographic concepts both for research and the clinic can include the fifth edition by Basmajian and DeLuca,5 as well as books by Loeb and Gans,7 Gnatz,8 and Kimura.9 Lastly, in consideration of EMG of the head and neck, the work of Faaborg-Anderson10 is a most worthy read. Although most of these citations are from older sources, this author considers them to be some of the best resources available for acquainting oneself with proper methodology.
Methods of Electromyography Recording
Whether referring to the older use of analogue systems, or the present use of digital systems in electromyographic signal acquisition and conditioning, the required equipment continues to consist of electrodes (various types of surface and intramuscular), as well as a preamplifier, amplifier, filter, display, and data storage unit. Electromyography units can be purchased in one of two ways: first, as an integrated system, which may come with unexpected limitations imposed by the system algorithms; and second, as a set of components, which will allow for greater flexibility but require development responsibility from the user. Systems used for diagnostic purposes are almost always integrated units, whereas those used in the laboratory are more frequently composed of individual components.
When an integrated system is to be used, it is important to consider a variety of factors. These factors include, but are not limited to, the type of electrode cable input, location of the preamplifier and amplifier, upper limits for amplification, limitations in sampling rate, site and type of filter, 3-dB down point of filter, and the site and extent of display smoothing. It is generally advisable to use a system that will record the full bandwidth of the amplified EMG signal and to store that signal onto the hard drive before the signal is filtered; this is to prevent the loss of information that was obtained during the acquisition process. However, antialiasing should be performed before the signal is sampled. Antialiasing is based on the Nyquist theorem that states that a signal must be sampled at no less than twice its frequency. If the sampling rate is too low, the signal will be incorrectly reconstructed and findings will be invalid. All systems do not meet the specifications for an intended project, and so it is up to the clinician/investigator to make a careful decision regarding appropriate application of an available unit.
Naturally, electrical safety aspects of EMG systems must be considered. Only equipment that is properly grounded and that has appropriate isolation built into the system is permitted for use in hospitals. Nonetheless, both component and integrated systems must be routinely examined in order to check for current leakage. Extension cords should never be used with EMG equipment, and patients should not be connected to other electrical devices during EMG testing. The safety of intramuscular EMG recording of the pharyngeal and laryngeal musculature was reinforced by a report from Mu and Yang.11 These investigators recorded intramuscular needle electrode EMG activity from the posterior cricoarytenoid muscle of 1200 patients, with no reported complications.
A primary decision to be made by clinicians and researchers is that relating to the type of electrodes to be used in a study. Investigations relating to various aspects of deglutition have been performed on humans as well as animals and on structures ranging from the lips through to the esophagus. These have been performed with surface, surface-suction, needle, hooked wire, monopolar, and bipolar electrodes. A description of the various types of electrodes and appropriate application of a particular type of electrode can be found in many sources within the literature.2, 3, 12, 13, 14, 15 At times, the type of electrode used for investigation has been a function of the level of scientific advancement at the time in which the study was performed as well as a function of the questions that were proposed. Therefore, even the best science from a particular period is influenced by the instrumentation of the time. Unfortunately, at times, it has also been a function of what the clinician had available. And so, a reader must evaluate the appropriateness of reported methodology before accepting the accuracy of reported results. For example, an EMG study using surface electrodes and in which the muscles of interest are deep to the surface is a study that is seriously open to question.
When EMG is performed for diagnostic purposes, concentric needle electrodes are, and generally should be, used. However, the use of needle electrodes and the use of monopolar electrodes are not the best selections for the study of the physiologic aspects of human deglutition. Because of the movements associated with deglutition, it is often quite difficult to execute a comfortable and normal swallow when a needle is placed within a muscle. An intramuscular EMG study with simultaneous recording from bipolar hooked wire electrodes and from a concentric bipolar needle electrode placed in the thyroarytenoid muscles of 10 adult canines compared the amplitude of activity during vagal stimulation. Signals from both electrode types showed similar complex action potentials. Furthermore, there was no reported difference in terms of electrode stability or vocal fold injury.16 Such reports reinforce the appropriate selection of hooked wire electrodes in the examination of a dynamic task such as deglutition. Also to be considered is the use of monopolar vs. bipolar recordings. A monopolar electrode records from a wide field, whereas a differential recording between two closely spaced (bipolar) electrodes records better from a more restricted area. Figures 1,2 to 3 are differential recordings obtained with bipolar, hooked wire electrodes.
Surface EMG is often used by investigators who are less concerned if the EMG activity is recorded from a group of muscles within a wide field, such as the submental region. Submental surface EMG is acceptable when the investigators are not interested in differentiating geniohyoid activity from that of the mylohyoid or digastric muscles. A commonly referenced textbook relating to the applications of surface EMG is that by Cram and Kasman.17 This book emphasizes that an appropriate use for surface EMG is for biofeedback, not as a diagnostic tool. The authors state that EMG biofeedback techniques fall roughly into three clinical categories: (1) systemic relaxation, (2) muscle strengthening or disinhibition, and (3) coordination. Coordination techniques are described as an advanced level of biofeedback and usually follow successful relaxation or strengthening therapy and is intended to help the patient learn how to obtain the correct balance of agonists/antagonists. Other sources for information relating to the use of surface EMG are DeLuca,18 Kasman et al.,3 and Merletti.19
It is important to stress that surface EMG is easy to use and equally as easy to abuse. Experienced electromyographers are quite aware that ease of use does not equate with acquisition of accurate information.20
Historical Background
According to Basmajian and DeLuca,5 the birth of the field of neurophysiology can be attributed to a 1791 experiment by Luiggi Galvani. Galvani developed his concept of "animal electricity" following a series of experiments in which he depolarized frog leg muscles by using metal rods to make contact with the leg muscles. A publication that is cited as reporting the first study of electromyographic signals was that by Piper.21 Other early and important work reported by Basmajian and DeLuca includes, but is not limited to, that of Gasser and Erlanger,22 Proebster,23 Adrian and Bronk,24 Kugelberg and Petersen,25 Denny-Brown,26 and Buchthal et al.27, 28
Electromyography has been used to study aspects of motor activity during swallowing for many decades. In 1956, Doty and Bosma29 described findings from an investigation performed on 28 animals from three species (cat, dog, monkey). In that report, the animals were anesthetized and a series of electrode placements began at the mylohyoid muscle and progressed caudally as far as the inferior pharyngeal constrictor; diaphragmatic activity was also recorded. The superior laryngeal nerve was stimulated to produce a swallow. The authors presented a detailed qualitative description of the reflexive stage of deglutition and the interaction of the muscles associated with the oral and pharyngeal stages of swallowing, and also provided a discussion of the similarities and differences across species.
The descriptive work by Doty and Bosma is still respected for its early contribution toward the understanding of, and interest in, the process of deglutition. Follow-up studies in other laboratories reported that the findings of Doty and Bosma were similar to their work with other mammals.30, 31, 32 A consistent pattern of contraction following muscle ablation was reported in a study of anesthetized dogs by Maeyama.33 Additionally, Maeyama concluded that the geniohyoid and thyrohyoid muscles were primarily responsible for laryngeal elevation; however, at that time, the author did not recognize the importance of these suprahyoid and infrahyoid muscles to safe oral intake. The early EMG studies of deglutition with both animal and human models are cited in the book by Basmajian and DeLuca.5
Other early and equally interesting EMG investigations examined activity in the striated and smooth muscles of the animal esophagus.34, 35, 36 As with the work of Doty and Bosma, these early esophageal studies were primarily descriptive; but they provided methodologic information that was important to the advancement of this field of study.
There are considerable esophageal EMG data reported from animal models; however, this review is directed toward work that has been reported on the adult human, and much of the swallowing research using the human model has been performed on muscles associated with the oral or pharyngeal stages of deglutition. This is reasonable because of the difficulty of inserting electrodes into the esophagus or lower esophageal sphincter of a human volunteer. However, Basmajian,37 cites a 1960 report by Petit, Milic-Emili, and Delhez in which diaphragmatic EMG was performed on a conscious man via the esophagus.
In an editorial article addressing the application of intraluminal suction electrodes to the esophagus, Zelter et al.38 stated five reasons why they believed that electromyographic recordings were difficult to perform for evaluation of esophageal function: (1) movement of the esophagus produced an artifact in the recorded signal, (2) occlusive mucous plugs could make it difficult to secure a strong fixation of suction electrodes to the esophageal wall, (3) electrical activity from the heart could interfere with the EMG signal, (4) reduced signal amplitude occurred because of extravasation of blood with subsequent reduction in the suction force, and (5) displacement of the electrode could easily occur due to insufficient suction. Given that these criticisms were published in 1990, it appears that investigators have resolved at least some of those issues. Intraluminal esophageal catheters have been reported in human subject research39, 40, 41, 42; additionally, intramuscular EMG research has been performed during surgical procedures.43
Because the lower esophageal sphincter (LES) is exposed to pressures changes within that region, gastroesophageal reflux can, and does, occur. In an effort to better understand the effects of intra-abdominal pressures on LES function, simultaneous pressure and intramuscular EMG recordings from the LES were obtained from 17 normal subjects who were scheduled for surgical repair unrelated to LES function. At the completion of the surgical procedures, the anesthetists were instructed to move the endotracheal tube so as to induce coughing and straining in the anesthetized patients. Electromyography activity in the LES during both coughing and straining showed a significant increase over resting activity; this was interpreted to suggest the LES had contracted in order to assist in the prevention of gastroesophageal reflux. The authors concluded that the straining-esophageal reflex is "deranged" in patients who experience gastroesophageal reflux disease (GERD).43
Much of the early EMG research with human subjects and relating to the muscles of the head and neck were originally performed by investigators from the disciplines of speech science and from otolaryngology.10, 44, 45, 46, 47 Few of these publications considered the actions of those muscles as they related to chewing or swallowing; nonetheless, the methodologic aspects of the research were strongly related to future studies of the oral and pharyngeal stages of deglutition.
Similarly, much of the early work relating to oral function was also performed by those in the area of speech science. Before long, dental researchers began to include EMG techniques into their work as well.48, 49, 50, 51, 52 A PubMed (www.ncbi.nlm.nih.gov/entrez) search suggested that even though EMG is used for human and animal studies of the esophagus, it is used less often for study of the esophageal stage of deglutition than for oral and pharyngeal stages of swallowing.
Electromyographic recordings from the submental region are often performed with surface electrodes. There are many publications in which surface electrodes were used, both properly and improperly. Therefore, the reader must use care in accepting the validity of reported data.
Anatomic Sites of Interest for Electromyographic Studies of Deglutition
Clinically, it is important to be able to interpret physiologic information on the anatomic sites that are important to the three stages of deglutition.53 The sites54 that are located above the upper esophageal sphincter are of relevance to the safe passage of a bolus during the oral and pharyngeal stages of deglutition. The locations of particular interest to effective and safe passage include the lips, muscles of the submental region, tongue, soft palate, hyoid bone, pharyngeal constrictors, vallecular spaces, epiglottis, thyroid cartilage, false and true vocal folds, arytenoid cartilages, pyriform sinuses, and upper esophageal sphincter.
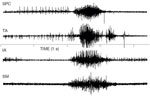
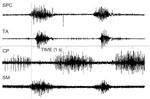
Simultaneous EMG recordings from five sites, including submental, pharyngeal, and laryngeal musculature, has indicated that the sequence of a normal swallow is preceded by contraction of the submental musculature followed by contraction of the superior pharyngeal constrictor, and then relaxation of the cricopharyngeus (CP) muscle. During the period of CP relaxation, both the thyroarytenoid muscles and the interarytenoid muscles contract, but EMG activity in the CP redevelops before the interarytenoid muscles relax. With a bolus as small as 10 mL, the thyroarytenoid muscle also continues to contract until after the CP has begun to close.55 Thus as one of several safety features, the CP segment will have separated the esophagus from the pharynx before the airway opens fully. Raw EMG data from these five muscles are displayed in Figures 4 and 5. Figure 4 shows an EMG recording from the superior pharyngeal constrictor, thyroarytenoid, interarytenoid, and submental muscles. Figure 5 shows EMG recording of two swallows from the superior pharyngeal constrictor, thyroarytenoid, cricopharyngeus, and submental muscles.
Figure 4: Simultaneous EMG recording from the superior pharyngeal constrictor (SPC), thyroarytenoid (TA), interarytenoid (IA), and submental muscles (SM) during swallow.
The SM was recorded with bipolar surface electrodes; all other muscles were recorded with bipolar hooked wire electrodes.
Figure 5: Simultaneous EMG recording of two swallows from the superior pharyngeal constrictor (SPC), thyroarytenoid (TA), cricopharyngeus (CP), and submental muscles (SM) during swallow.
The SM was recorded with bipolar surface electrodes; all other muscles were recorded with bipolar hooked wire electrodes.
An interesting review of brainstem mechanisms underlying the generation of sequential and rhythmic swallowing movements has been published.56 It analyzes the neuronal circuitry, the cellular properties of neurons, and the neurotransmitters possibly involved, as well as the peripheral and central inputs that shape the output of the network appropriately so that the swallowing movements correspond to the bolus to be swallowed. The mechanisms possibly involved in pattern generation and the possible flexibility of the swallowing central pattern generator are discussed. This review complements the anatomic and neurophysiologic information provided elsewhere.53, 57
Current Electromyography Research Related to Diagnosis and Treatment of Dysphagia
Clinically, EMG can be used to diagnose, describe, follow, or exclude diseases of the neuromuscular system. Thus, the clinician who is responsible for the diagnosis or treatment of dysphagia may work with a variety of patients who have received, or who may need, an EMG evaluation. The incidence of difficulty with deglutition among patients with neurologic and muscular diseases and disorders has been reported in many publications. A few publications are provided in the references58, 59, 60; however, the incidence outcomes should be reviewed with caution because the reported degree and scope of dysphagia may have been influenced by the questions asked by the clinical investigator. That is to say, the questions posed by a gastroenterologist may be more heavily weighted toward esophageal or gastric complaints, whereas the questions posed by an otolaryngologist or a speech-language pathologist may be more heavily weighted toward oral cavity or pharyngeal complaints. Thus, the incidence may be more than that which had been reported. Nonetheless, the likelihood of dysphagia among those with neuromuscular dysfunction is recognized across each of these disciplines, and interdisciplinary diagnosis and management are becoming increasingly common. Because of the natural bias incorporated into an area of clinical expertise, the need for clear communication across disciplines is imperative. Multidisciplinary organizations such as the Dysphagia Research Society (www.dysphagiaresearch.org) work to clarify terminology, to improve interdisciplinary communication and to encourage interdisciplinary research.
Additionally, the type of assessment that is employed may also bias the incidence outcome. For example, a patient with decreased sensation may be unaware of aspiration, and results from a questionnaire could be negative for dysphagia, whereas a cranial nerve (CN) examination indicating CN IX or X involvement and a videofluoroscopic examination would be positive for dysphagia and possibly for aspiration. Similarly, a history of aspiration pneumonia may prove negative for aspiration of oral secretions, but positive for aspiration of gastric secretions, and vice versa. Therefore, electrophysiologic assessment may provide definitive information when other means of examination have been inconclusive.
Although it has been frequently suggested by some that the swallow is safe if the gag is intact and if the vocal folds are functioning properly, there is every indication that such a statement is naive and generally inaccurate. The ability to intake food or liquid safely is far more complex than adequate vocal fold closure. Safe passage into the esophagus is more likely to occur when there is adequate hyolaryngeal elevation, adequate serial contraction of the pharyngeal constrictors, appropriately timed upper esophageal sphincter (UES) relaxation and velopharyngeal closure, than when there is complete vocal fold closure without the aforementioned events. If paresis or paralysis is suspected, the musculature associated with each of these events can be assessed with EMG. With adequate hyolaryngeal elevation the epiglottis will invert and provide a protective cover for the larynx.59 Furthermore, the larynx is moved out of the path of the descending bolus, and the inverted epiglottis helps to reduce the structural irregularity of the anterior wall of the pharynx. Thus, the pharynx begins to resemble a smooth tube, and a variety of structures are displaced in a series of closely coordinated activities.
Information relating to the EMG findings in specific muscles and muscle groups associated with deglutition is available for normal subjects as well as for several neuromuscular disorders. Most of the human studies of deglutition reported EMG findings from a single muscle or muscle pair including the buccinator, superior pharyngeal constrictor, palatoglossus, cricopharyngeus, and levator veli palatine.14, 60, 61, 62, 63, 64, 65, 66, 67 Reports from a single muscle or muscle pair do not provide information relative to the dynamics of the complex swallowing event. Therefore, studies that provide temporal information from a series of muscles in normal human subjects15, 68, 69 may often be useful for the process of integration of information from those studies performed on a single muscle in both normal and disordered populations.
To gain a better understanding of the most common deglutition complaints associated with neuromuscular diseases, Willig et al.70 surveyed 451 patients. They received responses from 409 patients (91%), which represented seven disorders. Difficulties with the oral stage were encountered in Duchenne muscular dystrophy (DMD), limb-girdle muscular dystrophy (LGMD), facio-scapulo-humoral muscular dystrophy (FSHMD), and spinal muscular atrophy (SMA). Difficulty opening the mouth was also noted in SMA. Certain features characteristic of specific diseases such as macroglossia in DMD and dryness of the mouth in dermatomyositis and polymyositis (DM/PM) and myasthenia gravis (MG) were also noted. Swallowing time was affected in MG, DM/PM, LGMD, and SMA. The overall prevalence of dysphagia in five disorders [SMA, myotonic dystrophy (MD), DM/PM, FSHMD, MG] was reported to be 34.9%. Given that these reports were from a survey, they are open to question. One can question if the lack of response was an indication of the absence of dysphagia, which would decrease the incidence percentage. Likewise, for those who did respond but indicated no difficulty, instrumental examination may have identified subclinical difficulties or even silent aspiration.
As would be expected, most of the EMG data on populations with neurologic impairment has been collected on diseases/disorders affecting the lower motor neuron system. Given that lower motor neuron dysfunction affects muscle function, impairment anywhere from the lips through the esophagus may occur and negatively influence an individual's ability to manage oral intake easily and safely. There are a variety of studies that support this relation between dysphagia and disorders of the lower motor neuron. Several examples are included below, but the list is not all-inclusive. Rather, only recent investigations specific to the relation with disordered deglutition are provided.
Myositis
Several investigations have documented dysphagia in patients with a diagnosis of dermatomyositis and polymyositis.71, 72 Myositis is an autoimmune disease characterized by muscle swelling and loss of function. Patients with myositis have been reported to have a limited relaxation of the cricopharyngeus muscle with elevated UES pressures.73 Differences in pharyngeal transit time from the oral cavity to the UES and abnormalities in cricopharyngeus muscle function (both hyperreflexive and hyporeflexive responses) have also been observed in patients with inclusion body myositis.74 Ertekin et al. concluded that EMG was prognostically useful for determining if a patient with this diagnosis would benefit from a cricopharyngeal myotomy. That is to say, a myositis patient with EMG findings of a hyperreflexive CP segment would be a more appropriate candidate for consideration of a CP myotomy than the patient with the same medical diagnosis but with a hyporeflexive CP segment.
The above statement relating to the decision to recommend CP myotomy is appropriate for patients with complaints of dysphagia secondary to a variety of medical diagnoses. Often, decisions are reached as a result of manometric findings. Other techniques may include EMG recording along with injections of solutions to temporarily relax the UES musculature. Whereas manometry provides information relative to pressure changes, EMG provides information directly related to muscle function.
One can hypothesize that manometry and simultaneous EMG recordings from the submental field and from the CP segment would prove prognostic. For example, if EMG revealed decreased submental muscle activity, it is likely that insufficient anterior-superior movement of the hyolaryngeal complex would occur; this would result in limited opening of the UES. In this case, if the CPEMG revealed appropriate UES relaxation, but there was insufficient hyolaryngeal displacement, the manometric pressure decrease may be negatively affected. Nonetheless, it is highly likely that this patient would not be a candidate for myotomy. Rather, the first therapeutic attempt would be to determine if such a patient could improve function of the submental musculature.
Myasthenia Gravis
A significant number of patients with the diagnosis of myasthenia gravis (MG) have been found to experience dysphagia.75 Myasthenia gravis is "most often, an acquired disorder that results in fatiguable [sic] muscle weakness, made worse by activity and improved with rest. It results from an autoimmune attack against the nerve-muscle junction. Other forms of the illness are caused by architectural changes of the nerve-muscle junction" (http://www.myasthenia.org). When 15 MG patients and 10 patients without MG were compared, all MG patients had dysphagia, which was improved, to varying degrees, following treatment with anticholinesterase and corticosteroid treatments.76 Submental EMG (SEMG) duration was significantly longer in MG patients, with and without dysphagia, than in non-MG patients and, as would be expected, the SEMG amplitude was lowest in MG patients with dysphagia. Interestingly, no abnormalities were observed in cricopharyngeus muscle activity of MG patients with (or without) dysphagia. Given decreased SEMG amplitude (suggestive of difficulties with oral transport into the pharynx) and given the positive effects of medications, clinical counseling with MG patients should include advising patients as to the best times to take medications relative to their preferred mealtimes. Some of what is lacking at this time are data relative to pharyngeal and esophageal muscle function before and after medications; such information would be useful in assisting in behavioral management.
Motor Neuron Disease
Amyotrophic lateral sclerosis (ALS) is the most common adult motor neuron disorder and, depending on the extent of lower motor neuron involvement, death generally occurs within 2 to 5 years following diagnosis. Amyotrophic lateral sclerosis presents clinically, with fasciculations, progressive weakness, muscle atrophy, and spasticity. In this author's clinical practice, patients who had been referred for complaints of dysphagia, and who, upon neurologic examination, were found to have early ALS, frequently presented with changes in speech. The most common concomitant speech changes included onset of hypernasality and/or imprecise articulation. In accordance with characteristics of this disease, these patients retained sensory input but were showing signs of motor weakness. Even for those patients who were able to manage the oral stage of deglutition, with disease progression the likelihood of silent aspiration was very high. Eventually each patient had to consider the option of tube feeding.
Diagnosis of ALS is supported by electrodiagnostic and laboratory testing, and along with assessing weakened musculature, EMG is used to help determine widespread involvement of muscles that are not symptomatic.77 An interesting monograph is available with sources relating to assessment of patients with ALS.78
In an effort to better understand the association between the CP muscle and the motor cortex, needle recordings of EMG responses were obtained from the CP during transcranial magnetic stimulation.79 Subjects were both healthy individuals and patients with a diagnosis of ALS and pseudobulbar palsy (PBP). Needle recordings obtained from the cricothyroid muscle of the larynx were also evaluated in six healthy subjects. Among other findings, in dysphagic patients with corticobulbar tract involvement and with pathologic and hyperreflexive EMG of the CP-sphincter muscle, the cortical motor evoked potentials (MEPs) of the CP muscle could not be elicited. The authors concluded that the latency of the MEPs and the central motor delay suggested a corticobulbar conduction pathway with few synaptic junctions to the CP motoneurons. The cause of the neurogenic dysphagia in ALS and PBP was determined to be related to the fact that the CP sphincter became hyperreflexic due to disinhibition, and the cortical MEP of the CP muscle disappeared due to degeneration of the corticobulbar pathway.
Post-Polio Syndrome
Approximately 25% of the 250,000 survivors of poliomyelitis experience the progressive muscle weakness known as post-polio syndrome (PPS).80 It is a clinical diagnosis that refers to the neuromuscular symptoms that begin to occur at least 15 years after stability in patients who had been previously diagnosed with acute paralytic poliomyelitis. The symptoms include (1) new weakness and atrophy in the limbs or bulbar or respiratory muscles [post-poliomyelitis muscular atrophy (PPMA)] and (2) excessive muscle fatigue and general fatigue with diminished physical endurance. Post-polio syndrome is a diagnosis that should not be made until other medical, neurologic, orthopedic, or psychiatric diseases that could explain the cause of the new symptoms have been excluded. Post-polio syndrome progresses slowly, and there are generally stable periods that can last for 3 years to as many as 10 years.81 When testing the accuracy of the diagnosis of PPS, macroelectromyography (macro-EMG) is useful for confirming progressive denervation and for excluding neuropathology.82 Progressive weakness and atrophy is believed to be the result of distal degeneration of residual motor units. Electrodiagnostic evidence of ongoing denervation includes fibrillation and fasciculation potentials on conventional EMG, increased jitter and blocking on single fiber electromyography (SFEMG), and smaller macro-EMG amplitudes in newly weakened post-polio muscles.83 Denervation continues in both clinically affected and unaffected muscles at a rate that is more rapid than that which occurs with the normal aging process.84
An EMG study of laryngeal function in PPS patients revealed findings consistent with similar studies in other skeletal muscles.80 Clinical experience has supported such findings in that patients with a history of bulbar polio have presented with dysphagia secondary to laryngeal and pharyngeal muscle weakness. Post-poliomyelitis patients may be able to swallow a barium capsule safely into the esophagus when the Mendelsohn maneuver85 is employed.
Muscle Paralysis
Another group of patients for whom EMG evaluation is appropriate are those who are suspected of experiencing muscle paralysis secondary to lower motor neuron injury or disease. For example, following a lateral medullary infarct, damage to the nucleus ambiguus is known to result in dysphagia.86 Although in Wallenberg's syndrome the lesion is unilateral, its effect on oropharyngeal swallowing is generally bilateral.87 Aydogdu et al. concluded that either a disturbance or a disconnection between the cells within the nucleus ambiguus or bilateral linkage to swallowing-related cranial motor neuron pools and to the contralateral nucleus ambiguus were responsible for the swallowing disorder. The EMG findings within both the larynx and pharynx have not been reported, but findings related to paralysis or paresis of these structures may prove to be of interest in the prognosis for recovery.
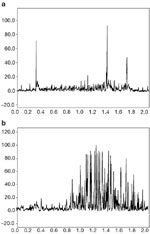
Electromyographic evaluation of laryngeal musculature is a frequently used diagnostic procedure performed in otolaryngology, but pharyngeal muscles are less frequently evaluated. Figure 6 is a rectified EMG recording (all negative values are squared and brought to the positive side of baseline) from the superior pharyngeal constrictor muscles of a patient who was referred due to a complaint of very severe dysphagia and a history of medullary stroke. The uppermost image is representative of the recording from the paralyzed pharyngeal constrictor, whereas the lowermost image is from the healthy contralateral side. Techniques for designing electrodes in order to obtain EMG recordings from the pharyngeal constrictors are described elsewhere.12, 14
Figure 6: Rectified EMG recording from the superior pharyngeal constrictor muscles of a patient with unilateral pharyngeal paralysis.
The upper image is recorded from the paralyzed side and the lower image is from the noninvolved side.
Scleroderma
A review of the literature relative to esophageal effects from scleroderma is available for the interested reader.88 Given that scleroderma is a connective tissue disease, it is not surprising that there are only a very limited number of articles in which EMG has been used with that patient population. Most often, the patient with scleroderma is evaluated with manometry and, on occasion, videofluoroscopy. However, two EMG studies were of interest to this author.
Patients with esophageal motor disorders and normal subjects participated in a study to determine if EMG activity obtained with suction electrodes within the esophagus was synchronous with manometric changes from the same esophageal site. In a patient with scleroderma and aperistalsis, vigorous EMG activity was observed. The EMG activity in the absence of simultaneous pressure changes was interpreted as a response of the longitudinal esophageal musculature to the swallow.89
In another investigation,90 simultaneous recordings of esophageal EMG and manometry were made in two groups of patients with scleroderma. One group was composed of patients who were experiencing dysphagia that had appeared recently, and who, on x-ray, had a normal-sized esophagus. The second group was composed of patients with a longstanding history of dysphagia and who were diagnosed with a dilated hypotonic esophagus. Five normal subjects served as a control group. The findings suggested that functional dysphagia of patients with scleroderma resulted from two different motor disorders. The first disorder was characterized by disorganized myoelectric hyperactivity with a possible manometric appearance similar to that of diffuse spasm. The second disorder was characterized by a marked decrease in myoelectric activity and corresponded "to the classic manometric finding of scleroderma involvement of the esophagus." One can question if the management of patients should differ as a result of the underlying characteristics of the disorder. If that is the case, then esophageal EMG may prove useful.
Conclusion
Electromyography is a well-established technique that is used to examine muscle activity both in basic science and clinical research as well as for clinical diagnosis. Diagnostic EMG can determine a site of pathology along the lower motor neuron, and it can be used in the differential diagnosis of various neuromuscular diseases. Understandably, within clinical medicine, EMG is more frequently used in neurology and otolaryngology than in gastroenterology. However, given the information that can be obtained from an electromyographic evaluation, use of the procedure may be appropriate for identification of muscle function that is negatively affecting oral intake.
This review discussed the history of EMG for both basic research and clinical purposes, the differences in EMG instrumentation and the strengths and weaknesses associated with the use of different instruments and electrode types, and the use of EMG in the study of certain neuromuscular disorders and diseases. Except for the historical review, the discussion has been restricted to adult humans.
Within the discipline of gastroenterology, there are a number of classic studies by highly respected investigators in which EMG was used with the animal model. The author apologizes for any apparent oversight that may have occurred due to the omission of those animal investigations, but she believes that the readership would be familiar with the well-known investigations.