Esophageal Anatomy
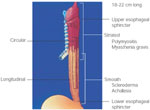
The esophagus is a tubular muscular structure that spans from the distal pharynx in the neck to the gastroesophageal junction below the diaphragm. The distal end of the tubular esophagus is normally in the abdomen and flares into the gastric fundus. Functionally, the esophagus extends from the lower border of the upper esophageal sphincter (UES) to the lower border of the lower esophageal sphincter (LES). Thus, although the UES is not a part of the esophagus, the LES is an integral part of it (Figure 1).
Figure 1: Esophagus: anatomic considerations.
Note that the cervical esophagus and the small part of the thoracic esophagus that includes the upper esophageal sphincter are composed of striated muscle. The lower two thirds of the esophagus, including the thoracic and abdominal parts containing the lower esophageal sphincter, are composed of smooth muscles. The diseases of the striated muscle include polymyositis and myasthenia gravis, whereas scleroderma and achalasia are diseases that involve the smooth muscle portion of the esophagus. (Source: AGA Gastroenterology Teaching Project, 2001, 2nd Ed. Unit 9, Esophageal Disorders, Slide 12, used with permission. Copyright © American Gastroenterological Association, Bethesda, MD)
Upper End of the Esophagus and Upper Esophageal Sphincter
The upper end of the esophagus is well defined by the lower border of the cricoid cartilage and the lower extent of the UES. The anatomic correlate of the UES is the cricoid cartilage anteriorly and the C-shaped fibers of cricopharyngeus on the sides and posteriorly. The cricopharyngeus is present in the lower part of the UES and oblique fibers of the inferior constrictor in the upper part. Anatomically, the UES is part of the pharynx and is appropriately termed the inferior pharyngeal sphincter. Thus, anatomically, the lower border of the cricoid cartilage and, functionally, the lower border of the UES define the upper end of the esophagus.
Lower End of the Esophagus and Lower Esophageal Sphincter
The lower end of the esophagus is defined anatomically by the junction of the esophagus with the stomach and, functionally, by the junction of the inferior border of the high-pressure zone of the LES compared to the lower pressure in the stomach.
Although the normal esophagogastric junction (EGJ) can be easily defined manometrically, its anatomic, radiologic, and endoscopic identifications are difficult and have been controversial.
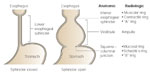
On barium swallow studies, the EGJ is difficult to identify in normal subjects because the swallow of contrast is associated with relaxation of the LES, which obliterates the EGJ. However, in patients with achalasia, the EGJ is marked by the lower end of a beak-like persistent narrowing of the LES and flare of the gastric fundus. This junction is often seen below the diaphragm. Rarely, a contracted LES may be identified in one of the many timed exposures during a normal barium swallow. During swallowing, in normal subjects, the LES is represented radiographically as the "ampulla" (corresponding to the vestibule described by anatomists) and the lower extent of the ampulla marks the EGJ. The ampullary segment is not seen when the EGJ is normally located below the diaphragm because it is not possible to fully distend this segment with barium because of the diaphragm and the intraabdominal location. However, when there is a hiatal hernia, the esophageal ampulla may be identified just above the hernia. In patients with a mucosal lower esophageal ring (Schatzki's ring), the EGJ is marked by this narrowing (Figure 2).
Figure 2: Anatomic radiographic landmarks of the lower esophageal sphincter (LES).
Normally, in between swallows, the LES remains closed. With a barium swallow the LES opens and its identity is lost unless some constriction lesions are present. For example, the anatomic inferior esophageal sphincter may be identified as a muscular ring (also known as contractile ring or A ring) or mucosal ring (also called Schatzki's ring or B ring) located at the squamocolumnar junction. Usually the squamocolumnar junction forms a zigzag line without a constriction in which case the lowermost part of the LES cannot be identified. Note that a relaxed LES can open wider than the esophageal body itself and has been called a vestibule by the anatomists and an ampulla by the radiologists. ESO, esophagus. (Source: Goyal RK. The lower esophageal sphincter. Viewpoints on Digestive Diseases. Am Gastroenterol Assoc 1976;8(3), with permission.)
On endoscopy, the muscular EGJ is hard to identify, and therefore reliance is placed on the squamocolumnar mucosal junction or the junction of the flat esophageal mucosal lining with the upper extent of the gastric mucosal folds. However, the squamocolumnar mucosal junction is often visualized as a zigzag line (Z-line). Therefore, the EGJ is hard to identify except when a straight-line squamocolumnar junction and a mucosal lower esophageal ring (Schatzki's ring) are present. Moreover, patients with short-segment Barrett esophagus have the lowermost part of the esophagus (i.e., the LES) lined by columnar mucosa, making endoscopic determination of the squamocolumnar junction an unreliable marker for EGJ. In such cases, the upper extent of the gastric folds may serve as a marker for the EGJ.
Based on the muscular anatomy, the EGJ is marked by a point where the tubular esophagus flares into the gastric fundus. However, this point is often difficult to identify. Other surrogate markers for the anatomic EGJ have been suggested, but none of them are fully satisfactory. Painstaking anatomic dissection of the muscles at the EGJ suggests that EGJ may be marked by the esophageal circular fibers on the right side of the esophagus that insert into the uppermost fibers of the gastric sling fibers on the left side.
The esophagus may be divided into three segments, namely the cervical, thoracic, and abdominal segments, based on their location. The esophagus is situated in the posterior mediastinum, behind the trachea and left mainstem bronchus, and it curves leftward to course behind the heart and in front of the aorta. It passes through an opening of the diaphragm called the esophageal hiatus that is located anterior to the opening for the descending aorta. Normally, the LES straddles the esophageal hiatus so that its upper half is present in the chest, whereas its lower half is located below the diaphragm in the abdominal cavity
Length of the Esophagus
Exclusive of the UES and the LES, the adult esophagus measures approximately 20 cm in length (range of 18 to 22 cm). Measured from the incisors, the esophageal body begins approximately at 18 to 20 cm, which marks the lower border of the UES and ends at approximately 40 cm (range of 26 to 50 cm). The esophagus is averagely shorter in women and ends at around 37 cm from the incisors (range of 22 to 41 cm). The terminal 2- to 4-cm region of the esophagus generally constitutes the LES.
Esophageal Wall
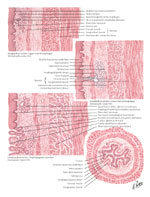
The wall of the esophagus, as in other parts of the gut, is made of the mucosa, submucosa, and muscularis propria (Figure 3). However, unlike the rest of the gut, the esophagus has no true serosal outer layer, but is covered by a thin and poorly defined layer of connective tissue. Normally, the mucosa of the esophagus is made of stratified squamous epithelium, except at the LES, where the squamous epithelium joins the gastric columnar epithelium. Compared to the rest of the gut, there are few glands, and its secretory function is limited. The muscularis propria is made of the inner circular and outer longitudinal muscle layers. There is also a less prominent layer of muscle oriented longitudinally and found between the mucosa and the muscularis propria called the muscularis mucosa.
Figure 3: Esophageal wall.
(Source: Netter medical illustration used with permission of Elsevier. All rights reserved.)
Cervical Esophagus (Striated Muscle Part of the Esophagus)
The muscularis propria is predominantly made of entirely skeletal muscle in the upper (cervical) part of the esophagus (approximately 2 to 6 cm of the proximal esophagus), and smooth muscle in the lower (thoracic) part of the esophagus. A variable length of mixed interdigitating muscle type is found in the midtransitioning portion of the esophagus, generally at the level of the carina (approximately starting 4 to 8 cm from the upper end, and extending approximately 10 to 13 cm from the lower border of the cricoid cartilage. Rarely, the entire esophagus may have striated muscle in its wall.
The longitudinal skeletal muscle fibers of the cervical esophagus originate from the superior aspect of the median ridge on the dorsal surface of the cricoid cartilage, and these fibers are joined by fibers from the cricopharyngeus and posterolateral cricoid cartilage laterally. The longitudinal fibers travel dorsally and caudally to meet approximately 3 cm below the cricoid cartilage, leaving a triangular area devoid of longitudinal muscle, called the Laimer's triangle.
It had been suggested that esophageal striated muscles develop from the smooth muscles by a process of cellular transdifferentiation. More recent studies, however, do not support this view. The striated muscles constitute the upper 2 to 6 cm of the proximal portion of the esophageal body.
The esophagus is innervated by both parasympathetic and sympathetic nerves. The parasympathetics control peristalsis via the vagus nerve. The medullary vagal postganglionic efferents arise from the lower motor neurons in the nucleus retrofacialis and the compact formation of the nucleus ambiguus. These nerves are myelinated, contain choline acetyltransferase and calcitonin gene-related peptide (CGRP), and make direct contact onto individual striated muscle fibers via the motor end plates. The main excitatory neurotransmitter at the motor end plate is acetylcholine, which acts on nicotinic cholinergic receptors. The role of CGRP is unknown. These nerve fibers to the striated muscles depart from the vagus in the upper part of the neck as the recurrent laryngeal nerve. Thus no cervical esophagus contraction can be elicited by electrical stimulation of the vagus nerve at the middle part of the neck. Moreover, peristalsis in the cervical esophagus can be abolished by a bilateral cervical vagotomy above the origin of the pharyngoesophageal branches.
The cervical esophagus also has a myenteric plexus with large number of neurons containing nitric oxide synthase (NOS) that are thought to receive preganglionic input from the dorsal motor nucleus of vagus (DMN). Nitric oxide may inhibit acetylcholine release from the motor end plates. The physiologic role of the nitrergic neurons in the striated muscle portions of the esophagus remains unknown.
Thoracic Esophagus (Smooth Muscle Part of the Esophagus)
The lower two thirds of the esophagus, including both the muscle layers, is composed of smooth muscles, which are thought to develop from transdifferentiation of the original striated muscle. The junctional segment that contains both striated and smooth muscles may be 4 to 6 cm long and is usually present around the level of the aortic arch. The junctional zone is the area with the weakest force of peristaltic contractions.
The esophageal smooth muscle (thoracic esophagus) is innervated by the extrinsic autonomic nerves and the intramural enteric nervous system. It has a well-developed myenteric plexus located between the circular and longitudinal smooth muscle layers of the lower esophagus. The vagal preganglionic motor efferents end onto motor neurons within the myenteric (Auerbach's) plexus. A second network of neurons in the submucosa (Meissner's plexus) also contains intrinsic afferents from the esophageal mucosa.
The preganglionic vagal fibers to the thoracic esophagus originate in the DMN. These preganglionic nerve fibers form the esophageal plexus and enter the esophagus at various levels. The fibers travel a few centimeters within the esophageal wall, and then connect to postganglionic neurons in the intramural plexuses.
The thoracic esophagus also receives sympathetic innervation from the postganglionic adrenergic neurons. The preganglionic sympathetic fibers originate in the intermediolateral cell columns of the T1–T10 spine and course into the cervical, thoracic, and possibly the celiac sympathetic ganglia. The postganglionic adrenergic fibers arising from these ganglia travel in the greater and lesser splanchnic nerves to terminate on the motor neurons in the myenteric plexus; most do not terminate on smooth muscle cells.
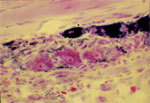
Esophageal myenteric plexus contains cell bodies of motor, sensory, and interneurons. There are two distinct populations of motor neurons. One set of motor neurons contains choline acetyltransferase (ChAT) and substance P and these neurons are called cholinergic neurons. Cholinergic neurons are excitatory in nature as they contract the smooth muscles by releasing acetylcholine. The second set of motor neurons is called nonadrenergic noncholinergic (NANC) inhibitory neurons, as they release a neurotransmitter that is neither adrenaline nor acetylcholine. These neurons have been shown to contain vasoactive intestinal peptide (VIP) and neuronal nitric oxide synthase (nNOS). They are called nitrergic neurons (Figure 4).
Figure 4: Neurons in myenteric plexus of human esophagus.
Note reduced nicotinamide adenine dinucleotide phosphate (NADPH) diaphorase staining neurons (dark areas) that are nitrergic in nature. Also note large neurons stained with eosin that may be cholinergic excitatory neurons. About 40% of the neurons in human myenteric plexus are nitrergic in nature.
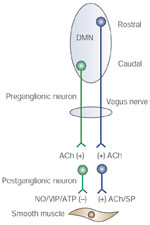
The excitatory (contractile) and inhibitory (relaxatory) motor myenteric neurons are innervated by separate sets of preganglionic vagal fibers. Thus, there are distinct excitatory and inhibitory pathways, each consisting of a chain of preganglionic fibers in the vagus and postganglionic neurons located in the myenteric plexus. The inhibitory vagal pathway nerves originate from neurons located in the caudal regions of the DMN. They may be recognized as the short-latency vagal efferents. These preganglionic neurons are cholinergic in nature and they synapse on the postganglionic nitrergic inhibitory neurons in the myenteric plexus. The excitatory vagal pathway nerves originate from neurons located in the rostral part of the DMN. They may be recognized as the long-latency vagal efferents. These preganglionic neurons are also cholinergic in nature and they synapse on the postganglionic cholinergic excitatory neurons in the myenteric plexus (Figure 5). The postganglionic myenteric motor neurons also receive input from local intramural sensory neurons that allows their activation by local stimuli. Thus the same myenteric motor neurons can participate in both local and centrally mediated (vagal) reflexes. Postganglionic sympathetic fibers also synapse on the myenteric motor neurons, but they have only a small effect of modulating the activity of the myenteric motor neurons.
Figure 5: Parallel inhibitory and excitatory innervation of the esophageal smooth muscle.
The excitatory pathway includes vagal preganglionic neurons that are located in the rostral part of the DMN in the brainstem. These fibers project onto the excitatory postganglionic neurons that contain acetylcholine (ACh) and substance P. The inhibitory pathway includes preganglionic vagal fibers that are located in the caudal part of the DMN. These fibers project onto postganglionic inhibitory neurons that contain nitric oxide (NO), vasoactive intestinal peptide (VIP), adenosine triphosphate (ATP), and substance P (SP).
The intrinsic afferent neurons have cell bodies located in the submucous as well as the myenteric plexuses. Their afferent endings are specialized sensors such as mechanoreceptors and chemoreceptors, and their efferent projections synapse on the myenteric interneurons. Some myenteric interneurons project orally whereas others project aborally. The orally projecting interneurons selectively synapse with the cholinergic excitatory motor neurons so that their activation causes muscle contraction above the site of the afferent stimulation. The aborally projecting interneurons selectively synapse with the nitrergic inhibitory motor neurons so that their activation causes muscle relaxation below the site of the afferent stimulation. The cholinergic neurons may also ascend orally before they innervate the muscle, and the nitrergic neurons may descend aborally before they innervate the muscle. Thus, activation of intrinsic afferents by stimuli such as balloon distention at any esophageal site produces reflex contraction above and relaxation below the site of the distension.
Sensory input from the esophagus to the central nervous system (CNS) travels via both vagal and splanchnic pathways. The vagal afferent pathway is involved in centrally mediated peristalsis, other vagovagal reflexes, and in the peripheral modulation of force and speed of peristalsis that is suited to the particular type of the food bolus that is transported through the esophagus.
The splanchnic afferent pathway may be primarily involved in esophageal nociception and other sensory phenomena. Sensory innervation of the esophagus is described in detail below (see Esophageal Sensations).
Lower Esophageal Sphincter
The terminal 2 to 4 cm region of the esophagus generally constitutes the LES. The LES is difficult to identify anatomically. The muscle in the region of the LES may be somewhat thickened, but this is not usually appreciable on anatomic studies. In humans, a thickened thin band of circular muscle called the inferior esophageal sphincter has been described in some anatomic descriptions. The inferior esophageal sphincter cannot account for the much wider functional LES. The inferior esophageal sphincter corresponds with the muscular ring sometimes seen in patients and may form the uppermost part of the LES. The lower part of the LES is constituted by the clasp fibers including the circular ring of muscle fibers on the right side and the top part of the sling fibers on the left side. The area between the inferior esophageal sphincter and the clasp fibers is the region of the LES. In humans, the LES is loosely fixed within the diaphragmatic hiatus partly by the phrenoesophageal ligament, which originates from the transversalis fascia of the diaphragm and inserts into the LES.
By electron microscopy, the muscles of the LES are of larger diameter and have fewer gap junctions compared to those of the adjacent esophageal body. These sphincter cells also manifest evaginations and irregular surfaces not seen in cells of the esophageal body, and possibly related to the tonically contracted state of these muscles. The LES is also distinguishable from the esophageal body by its greater intermuscular spaces and connective tissue, and greater concentration of mitochondria and smooth endoplasmic reticulum.
Muscle strips of the LES behave as tonic muscles, as compared to the adjacent phasic smooth muscle of the esophageal body. The LES develop spontaneous tone and contract slowly but steadily for prolonged periods with low energy expenditure. In contrast, the phasic muscles have little spontaneous tone and, upon stimulation, contract rapidly but briefly. These differences are due to differences in the contractile proteins and in the intracellular signaling pathways between the LES and the esophageal body circular muscle.
Functionally, the LES is best identified manometrically as a high-pressure zone that relaxes on swallowing. Barium swallow usually does not recognize the LES as it relaxes during swallowing. However, the LES correlates with the area radiographically called the "ampulla" or anatomically called the "vestibule." Mucosal rings can arise at its lower border, and muscular rings can arise at its upper borders (Figure 2).
The intramural myenteric motor neurons of the LES are similar to those described for the smooth muscle part of the esophagus. The vagal motor nerves provide parallel inhibitory and excitatory influences to the LES. The inhibitory vagal pathway nerves originate from neurons located in the caudal regions of the DMN. These preganglionic neurons are cholinergic in nature and they synapse on the postganglionic nitrergic inhibitory neurons in the myenteric plexus. The excitatory vagal pathway nerves originate from neurons located in the rostral part of the DMN. These preganglionic neurons are also cholinergic in nature and they synapse on the postganglionic cholinergic excitatory neurons in the myenteric plexus. The nitrergic inhibitory and cholinergic excitatory myenteric neurons that are postganglionic neurons in the vagal pathways also receive inputs from esophageal and gastric intrinsic sensory neurons. The projections from the esophageal sensory neurons mostly descend down to innervate the nitrergic inhibitory neurons, whereas projections from the gastric sensory neurons ascend to selectively innervate the cholinergic excitatory neurons to the sphincter. The myenteric motor neurons to the LES are also innervated by postganglionic sympathetic nerves. However, vagus nerves exert the main regulatory action on the LES, whereas sympathetic nerves may exert only a modulatory role.
The postganglionic excitatory and inhibitory neurons, apart from innervating the smooth muscle, also innervate the interstitial cells of Cajal (ICC). The ICCs also influence smooth muscle activity. Thus inhibitory and excitatory nerves may also influence smooth muscle activity indirectly via their actions on the ICC.
Diaphragmatic Sphincter
The diaphragmatic esophageal hiatus is encircled by a sling of striated muscle fibers called the diaphragmatic sling. This is innervated by branches of the phrenic nerve and also by the inhibitory motor fibers from esophageal nitrergic myenteric neurons. The tonic contraction of the diaphragmatic crural fibers may contribute to the basal LES pressure, particularly during the inspiratory contractions. These fibers relax during swallowing and transient lower esophageal sphincter relaxation (TLESR). They contract reflexively during episodes of increased intraabdominal pressure. The diaphragmatic sphincter behaves like an external LES.
Esophageal Physiology
Esophageal Body
Esophageal Transport by Gravity
In the resting condition, the esophageal body has a small amount of tone, but it is largely quiescent and may contain small amounts of air and reflect intrathoracic pleural pressures. Upon swallowing a bolus of liquid or barium in the upright position, the bolus is pumped back by the tongue into the pharynx. The bolus then travels quickly from the pharynx to the esophagus, and then through the esophagus into the stomach. The radiologic examination reveals that the head of a liquid barium bolus normally enters the stomach within a few seconds of initiating the swallow. This bolus transport through the esophagus is largely due to gravity. Such a fast movement of the liquid bolus does not occur in the supine posture. The tail of the bolus, however, is swept down by a progressive peristaltic contraction. Bolus of solid food also requires peristaltic contraction for its propulsion into the stomach. It takes approximately 8 to 10 seconds from initiation of the swallow to entry into the stomach (Figure 6). Thus, the head of the liquid barium bolus moves much faster than its tail in the upright posture, but the two move at about the same speed in the recumbent posture. Thus, careful attention to the movement of the tail of the bolus would be most relevant in assessing disorders of esophageal peristalsis.
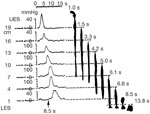
Figure 6: Simultaneous manometry and fluoroscopy of barium swallow in a normal subject.
Fluoroscopy shows that on swallowing, the barium column moves down the esophagus. Such barium movement occurs largely due to passive forces in the esophagus and is very marked in the upright position. The tail of the barium column moves down the esophagus by the peristaltic contraction. Note that the onset of peristalsis corresponds with the tail of the barium column. Also note that onset of peristaltic contraction at level 1 occurred at 8.5 sec after a swallow, at which time the barium column is pushed down to the lower 1 cm of the esophagus. (Source: Modified from Dodds WJ, Christensen J, Dent J, Arndorfer RC, Wood JD. Pharmacologic investigation of primary peristalsis in smooth muscle portion of opossum esophagus. Am J Physiol 1979;237(6):E561–E566, with permission from the American Physiological Association.)
Primary Peristalsis
Primary peristalsis is defined as a reflex esophageal peristaltic contraction wave associated with swallowing. It involves all phases of the swallowing reflex including the oral phase, pharyngeal peristalsis, UES relaxation, esophageal peristalsis, and LES relaxation. A marker of the start of the swallowing activity is contraction of the mylohyoid muscle, which is called the lead muscle of swallowing reflex. The peristaltic contractions are lumen-occluding contractions lasting 2 to 7 seconds that begin in the pharynx and move down the esophagus at a speed of about 4 cm/sec in the esophagus. The overall velocity of peristalsis in the esophagus is 4 cm/sec and it takes between 10 and 15 seconds to complete a primary peristaltic activity.
In the same individual and under the same conditions, the characteristics of the peristaltic wave remain rather constant on serial swallows and appear unaffected by aging. The esophageal peristalsis can be poor in premature infants. Several factors influence the amplitude, duration, and propagation velocity of the contraction wave in the esophagus: esophageal site; posture of the patient; consistency, size, and temperature of the food bolus; and resistance to the movement of the bolus.
The contraction amplitude is highest in the lower esophagus [69.5  12.1 mmHg, mean
12.1 mmHg, mean  standard error (SE)] and lowest in the mid-esophagus (35.0
standard error (SE)] and lowest in the mid-esophagus (35.0  6.4 mmHg). The area of lower pressure wave corresponds to the region of mixed striated and smooth muscles. The upper esophagus measures 53.4
6.4 mmHg). The area of lower pressure wave corresponds to the region of mixed striated and smooth muscles. The upper esophagus measures 53.4  9.0 mmHg. The duration of the contraction waves increases progressively in the distal parts of the esophagus. The propagation of the wave is fastest in the upper esophagus, and decreases in the middle and lower esophagus.
9.0 mmHg. The duration of the contraction waves increases progressively in the distal parts of the esophagus. The propagation of the wave is fastest in the upper esophagus, and decreases in the middle and lower esophagus.
The strength of contraction is less when the patient is upright compared to supine, and a liquid food bolus is associated with longer duration, stronger contraction, and slower propagation compared to a dry bolus of swallowed air. A larger bolus of food leads to stronger contractions. Warm boluses of food increase, whereas cold boluses decrease, the strength of contraction. The osmolality does not appear to affect the contraction wave. Increased abdominal pressure, as in the Valsalva maneuver, or strictures leading to outflow obstruction in the esophagus will slow the propagation of the contraction.
Although normal contraction waves generally sweep all material into the stomach, weaker contractions (generally less than 30 mmHg in the lower esophagus) may leave food residue behind. When there is outlet obstruction at the lower esophagus, or decreased esophageal wall compliance, resistance to the movement of the bolus (as indicated by increased intrabolus pressures) is increased, and a liquid bolus may flow back up through the ineffective contraction wave.
Secondary Peristalsis
Residual food in the esophagus, as seen with ineffective peristalsis, may be cleared by what is called secondary peristalsis. Secondary peristalsis does not involve full swallowing reflex. Instead it is a reflex that involves esophageal afferents and peristaltic activity restricted to the esophagus. It is not accompanied by pharyngeal contraction or UES relaxation. The amplitude and propagation speed of these contractions resemble those of primary peristalsis. Secondary peristalsis can be elicited experimentally by transiently distending the esophagus by a balloon in the lumen, or by air and water boluses. However, there are some differences in the ensuing secondary peristalsis depending on the region of the esophagus that was distended. The distention can also elicit a primary peristalsis.
In the striated muscle portion of the esophagus in animals, there is no difference between primary and secondary peristalsis other than association with a pharyngeal swallow and the method of initiation. Both are dependent on central vagal pathways. In humans, balloon distention in the esophagus may lead to a pharyngeal peristaltic wave that progresses from the striated to the smooth muscle portions of the esophagus.
In the smooth muscle part of the esophagus, secondary peristalsis is a local reflex elicited by local sensory nerves. It is similar to peristaltic reflex in the intestine. Distention of the esophagus causes activation of local sensory nerves that elicits contraction above the distention and relaxation below it. The contraction wave (secondary peristalsis) then progresses distally, moving the food bolus ahead of it.
Tertiary Contractions or Nonperistaltic Contractions
The term tertiary contraction is no longer used and it is not seen normally. In the past, it was applied to nonperistaltic contractions, which are contractions that lose their progressive character and occur simultaneously throughout the esophagus. On barium swallow, the nonperistaltic contractions may give the appearance of a corkscrew or a beaded esophagus.
Esophageal Propulsive Force
When the passage of a bolus of solid food is obstructed because of a mechanical obstruction in the esophagus, a sustained propulsive force that attempts to overcome this obstruction is exerted on the bolus. In humans, when a balloon is inflated in the esophageal lumen and anchored so that it is not allowed to migrate, a traction force of up to 200 g representing the esophageal propulsive force is generated on the balloon. This force is generated by contractions of the muscles above the balloon. The propulsive force increases with increasing size of the bolus and is greater in the lower than in the upper esophagus. While the balloon is anchored, or the food bolus is impacted, the esophagus distal to the balloon or the bolus remains relaxed by the distally projecting intramural inhibitory nerves. The sustained propulsive force is converted into the force of peristaltic contraction when the bolus is allowed to move or the experimental balloon is deflated. Muscle contractions producing sustained propulsive force may be responsible for the sensation of food sticking or dysphagia that may escalate into severe spasm-like chest pain. The esophageal propulsive force is reflexively mediated and involves both central and local afferent and efferent pathways.
Deglutitive Inhibition
The swallow-evoked peristaltic contraction consists of a wave of inhibition followed by that of contraction. The wave of inhibition that precedes peristaltic contraction is called deglutitive inhibition (Figure 7).
Figure 7: Demonstration of deglutitive inhibition in human esophagus.
In these studies intraesophageal pressure was artificially raised by inflating an intraluminally placed balloon, and esophageal responses to swallows were studied. In the left panel the balloon is 13 cm above the LES, and in the right panel the balloon is 8 cm above the LES. Swallows caused a fall in the artificially elevated esophageal pressures that were followed by peristaltic contraction. Note that the duration of swallow-induced fall in pressure at 13 cm is shorter than that at 8 cm. These studies show that the latency period before the onset of the peristalsis is in fact a period of inhibition, and that there is a gradient of increasing duration of deglutitive inhibition distally along the esophagus. (Source: Sifrim D, Janssens , Vantrappen G. A wave of inhibition precedes primary peristaltic contractions in the human esophagus. Gastroenterology 1992;103(3):876–882, with permission from the American Gastroenterological Association.)
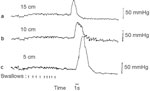
The phenomenon of deglutitive inhibition is essential for drinking of fluids at a rate faster than one swallow every 10 seconds. This is because the esophageal contraction in response to a single swallow lasts 8 to 10 seconds, and this will obstruct the bolus of a second swallow taken less than 8 second afterward. However, during the usual drinking of water, swallows may be accomplished every 1 to 2 seconds. This is made possible by the phenomenon of deglutitive inhibition in which a swallow abruptly inhibits any ongoing contraction in the esophagus. When multiple swallows are taken in rapid succession, the esophageal body remains inhibited until the last of the series of swallow, after which there is a fully conducted peristaltic contraction wave (Figure 8).
Figure 8: Diagramatic representation of manometric tracing demonstrating deglutitive inhibition.
Note that the subject is making repeated swallows every 1 to 2 seconds. During the swallows there is no activity in the esophagus. The last swallow was followed by a peristatic contraction.
Refractory Period
Esophageal peristaltic contractions are followed by a period of refractoriness during which the esophageal muscle is poorly responsive or nonresponsive to excitatory stimuli. The deglutitive inhibition and refractoriness may inhibit contractile responses to swallows that are taken at close intervals of less than 10 to 15 seconds apart.
Neuromuscular Control of Peristalsis
Cervical Esophagus
The distention-induced secondary peristalsis and esophageal propulsion in the striated muscle portion are mediated by central reflexes. The deglutitive inhibition in the cervical esophagus is thought to be centrally mediated by inhibiting lower motoneurons in the compact formation of the nucleus ambiguus. Whether the intramural NOS-positive neurons in the myenteric plexus within the striated muscle play a role in peristalsis and deglutitive inhibition is not known. It has been hypothesized that a central sequential firing of vagal motor neurons with independent nerve fibers running progressively distal in the esophagus (much like a distributor cap and wires of an automotive engine) leads to peristalsis in the striated muscle portion of the esophagus (Figure 9).
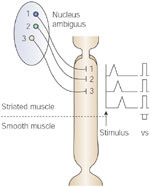
Figure 9: Peristalsis in striated muscle portion of the esophagus.
Note that swallow (stimulus)-induced peristalsis is due to sequential activation of lower motor neurons in the nucleus ambiguus in the brainstem. When the peripheral end of the decentralized vagus nerve is electrically stimulated (VS), all segments of the esophagus contract simultaneously.
Thoracic Esophagus
Primary peristalsis in the thoracic esophagus is also orchestrated by the swallowing program generator (SPG) in the brainstem. However, the mechanism of peristalsis in the smooth muscle segment is complex and involves coordinated activities of the vagal inhibitory and excitatory pathway, regional gradients of the myenteric inhibitory and cholinergic excitatory nerves, and the regional characteristics of the esophageal smooth muscle.
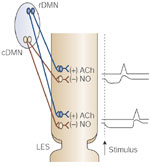
The peristalsis in the smooth muscle is based on the fact that the duration of the deglutitive inhibition associated with swallowing increases distally along the length of the esophagus. This gradient of inhibition is manifested only as the gradient of increasing latency of contraction in the noncontracted esophageal body smooth muscle. This gradient is due to both central and peripheral mechanisms. The central mechanism involves near-instantaneous activation of the inhibitory short-latency vagal fibers, which arise from neurons located in the caudal part of DMN. This is transmitted to all levels of the esophagus by the SPG so that the esophagus in its entire length is inhibited promptly on swallowing. The distally increasing inhibitory nerve influence is responsible for the distally increasing duration of inhibition along the esophagus. The myenteric inhibitory neurons were thought to act by releasing a NANC inhibitory neurotransmitter that is now shown to be nitric oxide (Figure 10). In addition, regional properties of the esophageal smooth muscle may also contribute to the distally increasing gradient of the duration of the deglutitive inhibition.
Figure 10: Central control of peristalsis in the smooth muscle portion of the esophagus.
Upon swallowing (stimulus), the inhibitory pathway neurons in the caudal DMN (cDMN) are activated first, which causes simultaneous inhibition of all parts of the esophagus. This inhibition lasts longer in the lower than in the upper parts. As the inhibition ends, sequential activation of excitatory (including cholinergic) neurons in the rostral DMN (rDMN) elicits a contraction wave that is peristaltic in nature.
The deglutitive inhibition is immediately followed by deglutitive excitation that is manifested by the esophageal contraction. Deglutitive excitation involves noncholinergic rebound excitation as well as cholinergic excitation.
Experimental studies have shown that stimulation of the NANC inhibitory nerves causes inhibition of the esophageal smooth muscle that is followed by a rebound contraction. The mechanism of rebound contraction is not known. It is clearly not cholinergically mediated. It is not clear whether the inhibitory transmitter itself somehow causes rebound contraction or an unknown NANC excitatory neurotransmitter is released after the release of the inhibitory neurotransmitter. The contribution of the noncholinergic rebound contraction to the force of esophageal peristaltic contraction increases distally along the length of the esophagus.
The deglutitive cholinergic excitation also follows the deglutitive inhibition and overlaps the rebound contraction. The cholinergic excitation involves activation of the excitatory vagal (long latency fibers) pathway consisting of preganglionic neurons in the rostral part of the DMN and postganglionic cholinergic neurons in the myenteric plexus. The sequential activation of the excitatory vagal pathway supplying the esophagus in a craniocaudal orientation leads to sequential wave of excitation that is timed to cause peristaltic contraction in the esophagus. There is a distally decreasing gradient of cholinergic innervation along the esophagus. As a consequence, cholinergic excitation provides greater contribution to the force of peristaltic contraction in the upper than the lower parts of the smooth muscle esophagus.
The swallow-evoked sequential cholinergic excitation is timed to occur when the deglutitive inhibition at different esophageal levels is terminated. However, there is overlap between the deglutitive inhibition and the deglutitive excitation. This overlap is most prominent in the proximal and least prominent in the distal parts of the esophagus. As a consequence, cholinergic deglutitive excitation causes greater shortening of latency of swallow-associated contraction in the proximal than in the distal parts of the esophagus. This cholinergic influence is maximal in the proximal portion of the esophagus, and decreases distally. The greater proximal cholinergic influence may contribute to a shorter latency of contraction proximally and progressively increasing latencies distally along the esophagus (Figure 11).
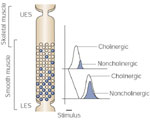
Figure 11: Gradient of cholinergic excitatory and noncholinergic inhibitory nerves in the smooth muscle portion of the esophagus.
The cholinergic excitatory innervation (open circles) is most marked in the proximal part and decreases gradually in the distal part. On the other hand, the inhibitory innervation (close circles) increases distally along the esophagus. As a result, upon stimulation the latency of contraction increases gradually distally along the esophagus, resulting in peristaltic sequence of contraction that is entirely located locally in wall of esophagus. (Source: Crist J, Gidda JS, Goyal RK. Intramural mechanism of esophageal peristalsis: roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci USA 1984; 81(11):3595–3599 with permission)
In conclusion, peristalsis in the esophageal smooth muscle is due to distally increasing duration of deglutitive inhibition followed by deglutitive excitation. The pattern of activation of the inhibitory and excitatory vagal pathways, the regional gradients of inhibitory and excitatory myenteric nerves, and the intrinsic properties of the smooth muscle all determine velocity of peristalsis. The esophageal peristaltic contractions themselves are a blend of noncholinergic and cholinergic components. As a consequence, cholinergic antagonists such as atropine increase the latency and decrease the amplitude of contraction in the proximal but not the distal parts of the esophagus. In contrast, antagonists of NOS reduce the latency mainly in the distal segments of the esophagus and lead to simultaneous contractions.
Nitric oxide (NO) produced from nNOS in the nerve terminals is the major inhibitory neurotransmitter in the esophagus. Nitric oxide causes inhibition by a cyclic guanosine monophosphate (cGMP)-dependent mechanism. Vasoactive intestinal peptide serves as an intermediate in enhancing electrical spike–induced augmentation of calcium influx and NO synthesis from nNOS. Adenosine triphosphate (ATP) is not involved as an inhibitory neurotransmitter in the esophagus.
Acetylcholine and substance P are the major excitatory neurotransmitters. In the circular smooth muscle, acetylcholine causes influx of calcium. Influx of calcium causes activation of the calmodulin-myosin light chain kinase pathway to cause contraction. Acetylcholine also acts via the rho kinase and protein kinase C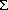 (PKC
(PKC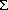 ) to cause contraction.
) to cause contraction.
Phasic Nature of the Esophageal Body Circular Smooth Muscle
The esophageal body is composed of smooth muscles that exhibit phasic muscle behavior. As such, it does not develop spontaneous tone and contracts transiently upon stimulation. The esophageal body circular muscles show less steep length-tension curves than the tonic muscle of the LES.
There are biochemical differences between the tonic and phasic muscles of the esophagus. Compared to the tonic LES muscle, the esophageal phasic muscle contains less  -actin, but a greater proportion of caldesmon, calmodulin, an acidic essential light chain (LC17a) and of a unique seven amino acid–inserted isoform of myosin. There are also regional differences in ion channels, which may account for electrophysiologic differences in the behavior of the esophageal body circular muscle. It has also been reported that there are regional differences in the ion channels along the length of the esophagus that may contribute to the regional differences in the contractility of the esophagus.
-actin, but a greater proportion of caldesmon, calmodulin, an acidic essential light chain (LC17a) and of a unique seven amino acid–inserted isoform of myosin. There are also regional differences in ion channels, which may account for electrophysiologic differences in the behavior of the esophageal body circular muscle. It has also been reported that there are regional differences in the ion channels along the length of the esophagus that may contribute to the regional differences in the contractility of the esophagus.
Lower Esophageal Sphincter
The LES remains tonically contracted at rest and relaxes or contracts further in response to various reflexes.
Basal Tone
Manometric studies show that the basal elevated pressures within the LES region are asymmetric: axially, the pressures are higher in the distal portion of the 2- to 4-cm length that constitutes the LES. Radially, although symmetric proximally, the distal part of the LES shows higher pressures on the left side, perhaps owing to both the diaphragm and the gastric sling fibers.
Respiratory variations in pressure are usually reversed between the upper and lower halves of the LES owing to thoracic and abdominal influences: in the upper half, inspiration causes a drop in pressure, whereas in the lower half, inspiration causes an increase in pressure. The respiratory fluctuations reverse at a point called the respiratory inversion point. This respiratory inversion point may extend over a region of about 0.5 cm and, in this region, respirations may cause biphasic pressure responses. The overall manometric pressure profile of the LES is complex because it is influenced by many dynamic events, including the up-and-down movement of the LES with respiration and swallowing.
The basal LES tone is determined by three factors: myogenic tone that is independent of any neural influences, cholinergic excitatory tone, and nitrergic inhibitory tone (Figure 12). The net balance between the myogenic, cholinergic excitatory, and nitrergic inhibitory tone determines the final basal LES pressure. As a consequence, the LES pressure may not change when all excitatory and inhibitory nerves to the LES are lost as in achalasia. However, selective antagonism of the cholinergic influences may cause a marked drop in the LES pressure due to unopposed action of the inhibitory nerves, and selective antagonism of the nitrergic inhibitory nerves may cause a marked increase in the LES pressure due to unopposed influence of the cholinergic excitatory nerves.
The myogenic tone of the LES is clearly identified by its behavior in vitro. The strips of LES circular muscle in vitro are readily distinguishable from the adjoining muscle strips from the esophageal body circular muscle. The LES muscle develops spontaneous tone when stretched, shows steep length-tension curves, and relaxes upon transmural nerve stimulation. The cellular mechanisms responsible for the myogenic tone are not fully understood. However, the LES muscle is normally depolarized and shows spontaneous spike activity that may cause continuous calcium ion influx. The depolarized state of the LES may be due to spontaneously open chloride channels. The composition of the contractile proteins also endows the LES with the ability to tonically contract at low levels of ATP utilization. Mechanical advantage derived from Laplace's law allows the sphincter to maintain large intraluminal pressures at low active contraction. It has been suggested that spontaneous production and release of prostaglandin F2 due to the presence of spontaneously active phospholipase C may provide stimulus for spontaneous tonic contraction of the LES. Cholinergic excitatory and nitrergic inhibitory nerves also exert a tonic effect on the LES; they act to augment or reduce the basal LES tone. The LES has lower levels of cytochrome c oxidase activity, and thus is more dependent on exogenous oxygen, which explains its sensitivity to anoxia.
due to the presence of spontaneously active phospholipase C may provide stimulus for spontaneous tonic contraction of the LES. Cholinergic excitatory and nitrergic inhibitory nerves also exert a tonic effect on the LES; they act to augment or reduce the basal LES tone. The LES has lower levels of cytochrome c oxidase activity, and thus is more dependent on exogenous oxygen, which explains its sensitivity to anoxia.
The intramuscular interstitial cells of Cajal (ICC-IM) are thought to play a role in transducing the effects of neurotransmitters released from nerve endings to smooth muscle cells. Animal models of ICC-IM deficiency and nNOS deficiency reveal that the former leads to an incompetent LES, whereas the latter leads to an achalasic LES. As such, chronic lack of ICC-IM may impair the myogenic function of the LES, but not necessarily impede the inhibitory neurotransmission.
Lower Esophageal Sphincter Relaxation
Generally, LES relaxes within 2 seconds after the onset of the swallow. The LES relaxes normally to a pressure very close to the intragastric pressure, and this relaxation lasts 8 to 10 seconds, followed by an aftercontraction, which is generally observed in the proximal part of the LES and is in continuity with the contraction wave of the esophageal body. The aftercontraction generally lasts 7 to 10 seconds. During the swallow-induced LES relaxation, there is inhibition of the spike activity. Lower esophageal sphincter relaxation is mediated via the nitrergic inhibitory pathway that uses nNOS. Genetic disruption on nNOS leads to failure of LES relaxation (Figure 13).
Isolated LES relaxation not accompanied by swallows or other esophageal motor activity can be elicited by applying pharyngeal tactile stimulation or experimentally by electrical stimulation of the superior laryngeal nerve using stimulus frequencies that do not elicit peristalsis. During a rapid succession of swallows, the LES remains relaxed and returns to baseline tone only after the last swallow. Lower esophageal sphincter relaxation either with or without swallows can be abolished by bilateral vagal section or cooling of the cervical vagus.
Esophageal distention at the level of either striated or smooth muscle segments can elicit LES relaxation. The reflex relaxation from distended striated muscle is centrally mediated and can be abolished by vagotomy, whereas the reflex relaxation from distended smooth muscle is mediated by the intramural nerves and is not abolished by vagotomy, although the vagus nerve may facilitate this relaxation.
Transient Lower Esophageal Sphincter Relaxation
Besides primary and secondary peristalsis, LES relaxation also occurs during belching, retching, vomiting, and rumination. Belching and vomiting are not associated with esophageal contractions. Rumination is associated with a reverse peristalsis. Continual manometric recordings revealed that in normal volunteers, most esophageal reflux episodes occurred during TLESR, were not associated with swallows, and were considered inappropriate. These transient relaxations were increased in patients with reflux esophagitis. Moreover, these relaxations were associated with reflux of gas, and are part of the belch reflux. Not all TLESRs were associated with reflux events, but reports vary on this correlation from approximately 10% to as many as 93% of TLESRs, depending on experimental conditions. The TLESR events appear to involve cholinergic vagal efferents acting on nitrergic postganglionic neurons. These relaxations are increased after gastric distention or in the presence of a nasogastric tube. Experimental studies suggest that vagal afferents arising from the stomach cause reflex LES relaxation via a vagovagal pathway that involves inhibitory vagal pathway neurons in the caudal part of the DMN and nNOS-containing neurons in the LES (Figure 14). Bilateral vagotomy or vagal cooling abolishes these relaxations. Serotonin may also play a role in the synaptic transmission, but antagonists do not affect the relaxation induced by electrical field stimulation of the LES inhibitory nerves. The TLESRs are associated with inhibition of spike activity and hyperpolarization of the muscle membrane.
Figure 14: Neural circuit for transient lower esophageal sphincter relaxation (TLESR) elicited by stimulation of subdiaphragmatic vagal afferents.
Subdiaphragmatic vagal afferents arising from the stomach project onto the nucleus tractus solitarius (NTS). The NTS neurons connect with neurons of the inhibitory pathway in the caudal part of the DMN. The inhibitory pathway neurons in the DMN mediate LES relaxation via nitrergic neurons in the LES.
Lower Esophageal Sphincter Phasic Contraction
The LES transiently contracts after the peristalsis-related relaxation. Lower esophageal sphincter pressure also increases with increased intraabdominal pressure, which is mediated via the excitatory vagal motor pathway. The distal part of the LES also contracts in phase with stomach contractions, and particularly during the late phase II and throughout phase III of the migrating myoelectrical complex (MMC).
External Lower Esophageal Sphincter: Diaphragmatic Crura
The crural diaphragm is composed of skeletal muscle and is innervated by the phrenic nerve. It arises from the esophageal mesenchyma during development, and probably involves transdifferentiation of smooth muscle. It augments LES pressure during inspiration. However, this behaves independent of the costal diaphragm so that, for example, during vomiting and eructations, the crural diaphragm is not contracted despite marked contraction of the costal diaphragm. The crural diaphragm is also inhibited during swallowing and TLESR. There is evidence to suggest that nitrergic postganglionic fibers may be mediating this inhibition of crural fibers.
Longitudinal Muscle
Although the circuitry is best characterized for circular smooth muscle of the esophagus, the longitudinal muscle also undergoes sequential excitation following a swallow. It shortens during swallowing and vomiting. It also contracts reflexively with esophageal acid perfusion, and this reflex contraction may be involved in the genesis of sliding hiatal hernia. Contraction of the longitudinal muscle may also be related to chest pain. The physiology and pharmacology of the esophageal longitudinal muscle are not well understood. However, it differs from the circular muscle in many respects. It contracts vigorously to substance P and shows a biphasic response consisting of relaxation followed by contraction in response to the nitric oxide donor, sodium nitroprusside.
Esophageal Sensations
Afferent fibers from the esophagus course along the vagal and splanchnic nerves. The vagal afferent fibers have their cell bodies in the nodose ganglion and project to the nucleus solitarius. Sympathetic afferents travel via dorsal root ganglia into the spinal cord at T1–L2. Afferent nerve endings are present in submucosa as perivascular and free nerve endings, in the epithelium as free intraepithelial nerve endings, and in the myenteric ganglia as intraganglionic laminar endings. The intraganglionic laminar endings may serve as mechanoreceptors; the intraepithelial nerve endings may serve as mechano-, thermo-, osmo-, and chemoreceptors in the esophagus. The afferent receptors are concentrated at the upper and the lower portions of the thoracic esophagus.
Esophageal mechanoreceptors also transduce painful sensations. Esophageal distention with a compliant balloon in awake subjects elicited three types of response depending on the degree of balloon distention. At small distention pressures, secondary esophageal peristalsis is produced without any sensory perception. At moderate distention pressures, the subjects experience pressure sensation only; but at high distention pressures, they experience chest discomfort and pain. Prolonged local distention may produce the sensation of lodged food or dysphagia. There are three types of sensory receptor. Each is connected to different sensory fibers. The sensory receptor and the afferent fiber constitute a sensory unit. The low-threshold mechanoreceptors have afferent fibers carried in the vagus nerves; they mediate esophageal reflexes and modulate spinal nociceptive pathways. The high-threshold mechanonociceptors have afferents in the splanchnic nerves; they mediate esophageal nociception. The wide dynamic range mechanonociceptors have afferents in the splanchnic nerves; they may transduce esophageal sensations.
Esophageal sensations, like the somatic sensations, are processed in the primary somatosensory cortex for sensoridiscriminative aspects and in the anterior cingulate cortex for affective motivational aspects. Parieto-occipital and midparietal cortices are activated by distention of the distal and proximal esophagus, respectively. Moreover, esophageal distention and acid perfusion induce spatially and temporally distinct patterns of cortical activation. Painful stimuli induce activation in the same cortical area as nonpainful stimuli and cause activation of anterior cingulate gyri. Mechanical and electrical stimuli of the esophagus also produce cortical evoked potentials. Sensory perception is necessary to evoke these potentials and they may be mediated via the wide dynamic range mechanosensitive afferents. Cortical evoked potentials may be used to determine the integrity of esophageal afferents.