Key Points
- Sensory disorders play a key role in esophageal functional disorders such as functional globus, heartburn, noncardiac chest pain (NCCP), and dysphagia.
- Convergence of primary sensory neurons from the esophagus and the heart on spinal dorsal horn neurons is responsible for overlapping features of esophageal and cardiac chest pain.
- Hyperalgesia is defined as heightened pain perception to painful stimulus, and allodynia as pain sensation to a stimulus that is nonpainful to normal subjects.
- Primary hyperalgesia is local (peripheral) and secondary hyperalgesia is more generalized (central) phenomenon. Both of these are included in under the term sensory hypersensitivity.
- Esophageal exposure to acid evokes sensory hypersensitivity.
- Esophageal primary sensory fibers are carried in the vagus and the spinal nerves. Most of the vagal afferent endings form two specialized structures: intraganglionic laminar endings (IGLEs) and intramuscular arrays (IMAs) in the esophagus.
- Esophageal primary afferent fibers have been classified as muscle tension-sensitive, mucosal mechano/chemosensitive and tension/mucosal receptors.
- The majority of these afferent fibers respond to mechanical and chemical stimuli. Therefore, they are polymodal in nature.
- The muscle tension-sensitive fibers in the vagus nerve have low thresholds for response and serve in physiologic reflexes. On the other hand, spinal afferent fibers may serve in nociception, because they encode the noxious intensity of stimulus.
- Acid excites primary sensory neurons in the esophagus by activating two proton-gated channels: transient receptor potential vanilloid-1 (TRPV1) and acid-sensing ion channels (ASICs).
- The activation of TRPV1 channel by acid can initiate neurogenic inflammation and release of proinflammatory substances from the tissue. Therefore, blocking the channel pharmacologically may alleviate esophagitis.
Clinical Disorders of the Sensory Nerves
Esophageal sensory nerves play a key role in esophageal functional disorders. Functional esophageal disorders (FEDs) represent chronic unexplained symptoms that have no detectable structural, inflammatory, or metabolic disease.1, 2, 3, 4, 5 Functional esophageal disorders include functional globus, heartburn, chest pain, and dysphagia. There is often an overlap of symptoms of each of these conditions. Functional chest pain, commonly known as noncardiac chest pain (NCCP), is a very common diagnosis in this patient population. The pain is easily confused with cardiac angina. About 15% to 30% of angiograms performed for chest pain are normal, and coronary artery spasm does not explain the symptoms.3, 4 In the United States, at least 500,000 coronary angiograms are performed annually for chest pain; functional disorders may be present in 75,000 to 150,000 of these patients.3, 4 Physiologic mechanisms underlying the symptom production are poorly understood. Clinical studies have demonstrated that patients with NCCP have lower thresholds for pain in response to esophageal distention (ED).6 It has been speculated that the etiology of NCCP involves either hypersensitivity of the sensory afferent fibers (i.e., peripheral sensitization) or the sensitization of central neurons in the spinal cord and brain (i.e., central sensitization).
Observations on Sensory Functions in Humans
The human esophagus is sensitive to several stimuli including mechanical (balloon distention and high-amplitude peristaltic contraction), chemical (acid-related heartburn), and thermal (cold and hot fluid-induced chest pain) stimuli under normal conditions. Esophageal pain is usually retrosternal in location and may resemble cardiac chest pain. This resemblance is due to convergence of sensory afferent fibers from the heart and esophagus in the same spinal dorsal horn neuron in the cervical and thoracic spinal cord.7, 8, 9
Hyperalgesia, Allodynia, and Hypersensitivity
Esophageal acid exposure may initiate visceral hyperalgesia, allodynia, secondary hyperalgesia to other areas of the viscera, and referral pain in the somatic structures.10, 11, 12, 13, 14, 15, 16, 17, 18 Hyperalgesia is a state in which the patient reports exaggerated pain to painful stimulus, whereas allodynia is the state in which the patient perceives pain to a stimulus that is generally nonpainful to normal healthy subjects. It is to be noted that hyperalgesia can exist without allodynia, but in most cases allodynia is associated with hyperalgesia. The term esophageal hypersensitivity is often used to indicate exaggerated pain response. However, this term also includes other abnormal phenomena such as esophageal hypermotility, dysmotility, or excessive acid reflux.
Overlap of Sensory Experience to Different Modes of Stimuli
Studies in humans indicate that the esophagus can sense mechanical, electrical, and thermal stimuli.17, 18 In healthy volunteers, nonpainful ED produces a pressing sensation in the chest, whereas a high intensity of distention produces pressing, pricking, and warm sensations. Electrical stimulation evokes pricking, shooting, and warm sensations in the chest. Subjects can discriminate cold and hot temperature by describing squeezing or pricking pain to cold (7°C) and burning, pricking and pressing pain to hot (50°C).17, 18 These observations clearly indicate that there is overlap in the nature of sensations to different modes (mechanical, electrical, and thermal), of stimuli suggesting polymodal properties of sensory afferents signaling to the central nervous system.
Acid-Induced Hypersensitivity
Studies in humans have consistently demonstrated that acid can alter the sensory perception of the esophagus.14, 15, 17, 18, 19, 20, 21 It has been shown that healthy volunteers can develop mechanical and heat hyperalgesia after prolonged acid exposure.14, 15, 19, 20, 21 Similarly, NCCP patients who have mechanical hyperalgesia to ED exhibit chemical hyperalgesia to acid exposure.1, 2 Interestingly, there is a gender difference in pain perception in the esophagus.21 Males exhibit significantly greater sensitivity to distention compared to females after acid perfusion. Males have a lower tolerance for acid perfusion volume compared to females. Although both males and females develop secondary somatic hyperalgesia following acid perfusion, females develop a significantly larger referred pain area in the somatic structure than males.
Primary and Secondary Hyperalgesia: Peripheral and Central Sensitization
Most of the psychophysical studies indicate that acid-induced hypersensitivity has two main components: primary hyperalgesia manifested immediately at the site of acid exposure and secondary hyperalgesia occurring in other areas of the organ not exposed to acid and in the somatic structures of the same dermatome that persists long after acid exposure.14, 15, 20, 21 It is thought that primary hyperalgesia occurs owing to hyperexcitation of primary sensory neurons, whereas the development of secondary hyperalgesia in other areas occurs owing to sensitization of neurons in the spinal cord.14, 15 The mechanism of sensitization in the central nervous system (CNS) is complex and diverse.22, 23 In the spinal cord, sensitization is generally manifested as an increased response of neurons to a variety of inputs following intense or noxious stimuli.24 Neurons undergo functional changes under the influence of multiple neurotransmitters, which leads to a heightened response of the neurons to nonpainful and painful stimuli. In short-term sensitization, as in the case of acute acid exposure (30 to 40 minutes), unmyelinated C-fiber activation lasting 1 to 2 hours may lead to a widespread increase in cell responsiveness in the CNS without any change in synaptic connections.24 In long-term sensitization, when central neurons receive excessive volleys of impulses for relatively long period of time, there are morphologic changes including synaptic connections, alteration of neural network, and decrease in inhibitory 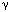 -aminobutyric acid (GABA)-ergic interneurons in the spinal cord.24, 25, 26 It is important to note that a change in cell responsiveness in the CNS may occur in a relatively short period (30 to 40 minutes) of increased inflow from the primary afferent fibers, but this change does not produce a long-term permanent neuroplastic change of the neurons. This phenomenon has been observed in most of the psychophysical studies.14, 15, 17, 18, 19, 20, 21 As indicated before, central sensitization occurs under the influence of several neurotransmitters released at synapses in the spinal cord. The most important of them are excitatory amino acid glutamate, substance P, calcitonin gene-related peptide (CGRP), prostaglandins, and adenosine triphosphate (ATP). Although the exact cellular mechanism still remains to be elucidated, it is at least known that glutamate and prostaglandin E2 are involved in acid-induced visceral hyperalgesia.27, 28
-aminobutyric acid (GABA)-ergic interneurons in the spinal cord.24, 25, 26 It is important to note that a change in cell responsiveness in the CNS may occur in a relatively short period (30 to 40 minutes) of increased inflow from the primary afferent fibers, but this change does not produce a long-term permanent neuroplastic change of the neurons. This phenomenon has been observed in most of the psychophysical studies.14, 15, 17, 18, 19, 20, 21 As indicated before, central sensitization occurs under the influence of several neurotransmitters released at synapses in the spinal cord. The most important of them are excitatory amino acid glutamate, substance P, calcitonin gene-related peptide (CGRP), prostaglandins, and adenosine triphosphate (ATP). Although the exact cellular mechanism still remains to be elucidated, it is at least known that glutamate and prostaglandin E2 are involved in acid-induced visceral hyperalgesia.27, 28
Studies in Experimental Animals
Sensory Innervation of the Esophagus
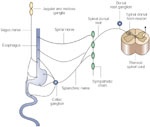
The esophagus is dually innervated by the primary sensory afferents that are carried in the vagal and the spinal nerves. A majority of these neurons are pseudounipolar cells. The cell bodies of the vagal afferents are located in the nodose and jugular ganglia and those of the spinal afferents are located in thoracic and cervical dorsal root ganglia (Figure 1). The vagal afferents to the upper one third of the esophagus are carried in the superior laryngeal branch of the vagus and the main vagal trunk. Vagal afferents to the lower thoracic, abdominal esophagus, and lower esophageal sphincter (LES) are carried in the vagal branches.29, 30, 31, 32, 33 The spinal afferents are contained in the thoracic splanchnic nerves that project onto the cervical (C1) to upper lumbar (L2) segment of the spinal cord.31, 33, 34, 35
Structures of the primary afferent endings innervating the esophagus have been studied by injecting tracers, including horseradish peroxidase (HRP), wheat germ agglutinin horse radish peroxidase (WGA-HRP) and passive carbocyanine dye (DiI), into the nodose or dorsal root ganglia.33, 36, 37 In the most oral part of esophagus, at the level of the cricoid cartilage, these labeled axons are abundant in the submucosa and mucosa and some fibers terminate in the epithelium.37 However, the innervation becomes progressively sparse in the thoracic and abdominal esophagus. The most densely labeled cells are consistently found between the muscularis externa and the interna along the whole length of the esophagus.
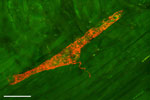
Electron and confocal microscopy has revealed that a majority of the vagal afferents terminating in the myenteric ganglia make a specialized laminar structure that encapsulates superficial and deep myenteric ganglia (Figure 2). These special terminal structures are known as intraganglionic laminar endings (IGLEs), which were first described by Nonidez38 and later by Rodrigo et al.39 One vagal afferent axon may divide and end in several IGLEs. The exact functional role of IGLEs was debatable for many years. It was speculated that these endings might act as tension-sensitive afferents.40 A study that combined electrophysiologic recording and injection of the tracer dye in the same fiber has revealed that the majority of the tension-sensitive afferents are IGLEs.41 The ubiquitous distribution of IGLEs in the gastrointestinal (GI) tract suggests that these endings detect passive (i.e., during filling) and active (i.e., during contraction) tension of the hollow viscera. Although the response characteristics of IGLEs of different species are very similar, their neurochemical profile is different between the species and strains. For example, in C57Bl/6 mice, only 50% of IGLEs have the P2X2 subtype of the ATP receptor,42 whereas in BalbC mice and guinea pigs 100% of the IGLEs are P2X2 receptor positive.43, 44 The IGLEs from mice show little or no immunoreactivity to the calcium-binding protein calretinin,43, 45 whereas in rat 80% of IGLEs are calretinin positive.46 Similarly, 100% of mice IGLEs express the vesicular glutamate transporter-2 (VGLUT2), but this is not so in rats.42, 47 What the physiologic implications of differential expression of neurotransmitters and receptors in different strains of mice and between the species are not known.
Figure 2: Confocal photomicrograph of anterogradely labeled intraganglionic laminar ending (IGLE) in the proximal striated part of the esophagus of the rat.
The IGLE was labeled by injecting DiI tracer dye into the nodose ganglion. Scale bar = 50  m. (Unpublished observation by W.L. Neuhuber and H.-R. Berthoud.)
m. (Unpublished observation by W.L. Neuhuber and H.-R. Berthoud.)
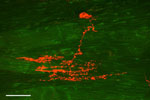
Besides IGLEs, there is another type of vagal axonal ending that innervates the smooth muscle. One axon traverses through the myenteric ganglia, enters at either the longitudinal or circular muscle layer, and makes a branching array parallel to the contractile elements. These endings are called intramuscular array (IMA) endings48 and they are mostly present in the smooth muscle segment of the lower esophagus, stomach, and small and large intestine. Interestingly, IMAs are more strategically located in the sphincters. In the esophagus, these endings are mostly restricted to the LES (Figure 3).49, 50, 51 These neurons maintain a close network with interstitial cells of Cajal (ICCs), and it appears that ICCs serve as a trophic function for survival of these fibers.52 Because of the IMA fibers' structural pattern and strategic location in the GI tract, some investigators believe that they are functionally in parallel stretch-sensitive endings, which primarily detect the changes in the length of the muscle.40
Figure 3: Confocal photomicrograph of a vagal intramuscular arrays (IMA) nerve terminal in a tangential section of the lower esophageal sphincter of a rat.
There is one striated muscle fiber on the left side of the figure; however, all the other fibers are smooth muscles. Therefore, it indicates that this ending is located at the transition zone of striated and smooth muscle of the esophagus. It is to be noted that in rats, IMAs mostly innervate the smooth muscle portion and lower esophageal sphincter (LES) of the esophagus. IMAs are rarely seen in the striated part of the esophagus. Scale bar = 50  m. (Source: Courtsey of Winfried L. Neuhuber.).
m. (Source: Courtsey of Winfried L. Neuhuber.).
Unlike vagal afferent fibers, the spinal afferent fibers are relatively understudied. Anterograde tracing by injecting cholera toxin B conjugated-HRP into the thoracic dorsal root ganglia (DRG) of the cat revealed selective labeling of the neurons in the esophagogastric junction.31 Two types of afferent fibers were found: fibers running in myenteric connectives in isolated bundles, and fibers within the myenteric ganglia. Within the myenteric ganglia, afferents produced axosomatic contact with myenteric neurons. In the interganglionic area, there were abundant unmyelinated afferent fibers containing few vesicles and mitochondria. The axolemma or endings of these afferents were thought to function as mechanoreceptors and release of neurotransmitter, which could act on myenteric neurons.31 Although a recent study has documented IGLE-like endings of the spinal afferents innervating the colon of guinea pig,53 existence of such endings has not yet been documented in the esophagus.
Functional Characteristics of Primary Afferents
Afferent fibers in the esophagus have been classified as mucosal receptors and muscle receptors on the basis of their termination in different tissue layers. This organization was inferred initially from the evidence obtained in electrophysiologic studies, by noting the pattern of response of fibers to intraluminal distention, mechanical probing of the mucosa, or instillation of local anesthetic into the lumen. Afferent fibers of each category have also been subclassified on the basis of sensory modality as mechano-, chemo-, or thermoreceptors. Although this scheme of subclassification is being commonly used, many afferent fibers can respond to more than one mode of stimulus (i.e., polymodal).
Muscle afferent fibers in the esophagus respond to intraluminal distention and generally exhibit slow adaptation to distention.29, 30, 54, 55, 56, 57, 58, 59, 60, 61, 62 Because these fibers respond to a change in muscle tension, they are also commonly known as tension receptors or tension-sensitive afferent fibers. These fibers are carried both in the vagus as well as the spinal nerves, and the majority of them are unmyelinated C-fibers or thinly myelinated A -fibers.63
-fibers.63
Afferent fiber response to isobaric phasic distention consists of three major patterns during and immediately after distention: a dynamic response or initial burst at the beginning followed by a tonic firing and an abrupt pause in firing after termination of distention. The initial high frequency of discharge is likely due to initial high tension that develops during active resistance offered by smooth muscle and also to local excitatory reflexes produced by intrinsic nerves in the myenteric and submucous plexi. The firing then declines owing to partial relaxation of the smooth muscle, but maintains a tonic level throughout the period of distention. Termination of the distending stimulus typically stops the firing of the fiber abruptly, owing to sudden loss of muscle tone, but firing resumes with the slow return of tone (Figure 4).
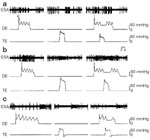
Figure 4: Intensity-dependent response of a vagal afferent fiber to graded esophageal distention (ED, 5–80 mmHg, 30 seconds).
The fiber had ongoing spontaneous firing (15 impulses/s) with a balloon placed in the distal esophagus. In each panel, the top trace illustrates the nerve activity represented as frequency histogram (1-second bin-width), the middle trace is nerve action potentials, and the bottom trace illustrates distending pressures. The fiber exhibited increasing firing to pressure up to 40 mmHg. However, the firing frequency did not increment at greater distending pressures (60 and 80 mmHg). The fiber exhibited a pause in firing after the end of distention (10–80 mmHg).
Vagal afferent fibers are essentially a homogeneous population having low thresholds (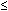 5 mmHg) for response to intraluminal distention.29, 56, 57, 58, 59, 60, 61 These fibers also respond to longitudinal stretch of the muscle and to electrically evoked contraction of the esophagus, suggesting their role in reflex regulatory functions in swallow-induced peristalsis.58 In vitro recording from vagus nerve-esophagus preparations have documented similar response characteristics of muscle afferents to circular muscle stretch.41, 64, 65, 66 Owing to their low threshold for response to mechanical stimulus, some afferents, but not all, exhibit rhythmic firing synchronized to change in intrathoracic pressure.57, 58
5 mmHg) for response to intraluminal distention.29, 56, 57, 58, 59, 60, 61 These fibers also respond to longitudinal stretch of the muscle and to electrically evoked contraction of the esophagus, suggesting their role in reflex regulatory functions in swallow-induced peristalsis.58 In vitro recording from vagus nerve-esophagus preparations have documented similar response characteristics of muscle afferents to circular muscle stretch.41, 64, 65, 66 Owing to their low threshold for response to mechanical stimulus, some afferents, but not all, exhibit rhythmic firing synchronized to change in intrathoracic pressure.57, 58
Although the muscle afferent fibers in general respond to intraluminal distention, there are some distinct differences in response patterns of vagal and spinal muscle afferents. The majority of vagal afferents in most species exhibits relatively high spontaneous firing (approximately 5 to 40 impulses/sec) with the only exception in sheep.67
The spontaneous activity of vagal afferent fibers supplying the LES is significantly greater than that of afferent fibers supplying the body of the esophagus. This is related to the high basal tone of the LES. Another distinctive feature of LES afferent fibers is that balloon distention 5 to 10 cm above the LES reduced the discharge of the neuron below its baseline resting activity. The reduction in activity was related to reflexive relaxation of the sphincter.29 The resting activity of LES afferent fibers in many instances is modulated by cardiorespiratory cycles.54, 55, 57, 67 In comparison, spinal afferents having low ongoing spontaneous firing (approximately 0.28 to 18 impulses/s) do not exhibit rhythmic firing during the cardiorespiratory cycle.30, 59, 60
Response characteristics of vagal afferents to graded phasic distention differ from spinal afferents. In the opossum, the vagal afferent fibers demonstrate a steep increase in activity within a narrow range of pressure (5–30 mmHg), but responses plateau at greater intensities (>40 mmHg) of distending pressures. A similar result in the ferret has been reported to step-distention (0.5–3 mL) where a majority of the afferents reach the plateau at the distending volume of 1 mL. In the guinea pig, nodose A-fibers are exquisitely sensitive to intraluminal distention and 70% of them reach a plateau 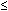 30 mmHg.68 However, the nodose C- and jugular A- and C-fibers exhibit a linear increasing response to distention up to 100 mmHg. The thresholds for responses of muscle fibers have been determined in the ferret (5–15 mmHg),69 dog (3 mmHg),56 opossum (1 mmHg),58 rat (<5 mmHg),57, 70 and guinea pig (4–10 mmHg).68
30 mmHg.68 However, the nodose C- and jugular A- and C-fibers exhibit a linear increasing response to distention up to 100 mmHg. The thresholds for responses of muscle fibers have been determined in the ferret (5–15 mmHg),69 dog (3 mmHg),56 opossum (1 mmHg),58 rat (<5 mmHg),57, 70 and guinea pig (4–10 mmHg).68
The response characteristics of spinal muscle afferents have not been studied extensively in many species. Comparative studies in the opossum reveal that spinal muscle afferents respond differently to graded intensities of distention. Unlike vagal afferents, spinal muscle afferents can have either low (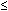 5 mmHg, 63%) or high thresholds (
5 mmHg, 63%) or high thresholds ( 30 mmHg, 37%) for response.58, 59 The low-threshold afferents exhibit a linear increasing response to a wide range (5–100 mmHg) of distending pressure,59 as the high threshold afferents respond to a distending pressure
30 mmHg, 37%) for response.58, 59 The low-threshold afferents exhibit a linear increasing response to a wide range (5–100 mmHg) of distending pressure,59 as the high threshold afferents respond to a distending pressure  30 mmHg (Figure 5). The intensity encoding properties of these two types of afferents to graded intensities of distention pressures indicate their possible role in nociceptive transmission to the spinal cord.
30 mmHg (Figure 5). The intensity encoding properties of these two types of afferents to graded intensities of distention pressures indicate their possible role in nociceptive transmission to the spinal cord.
Figure 5: Comparison of mechanosensitive properties of vagal and spinal afferent fibers innervating the esophagus of opossum (Didelphis virginiana).
a: Stimulus-response functions of three classes of afferent fibers to graded distention of the esophagus. The low-threshold mechanosensitive (LTM-vagus, open circles) fibers in the vagus nerve encode stimuli in the physiologic range and exhibit a saturation of firing at greater intensity ( 40 mmHg). However, in the spinal nerve, there are two distinct classes of mechanosensitive afferent fibers. A proportion of low threshold intensity-encoding fibers (LTM-spinal, open triangle) respond to innocuous to noxious intensity of stimuli, whereas some fibers have high thresholds (
40 mmHg). However, in the spinal nerve, there are two distinct classes of mechanosensitive afferent fibers. A proportion of low threshold intensity-encoding fibers (LTM-spinal, open triangle) respond to innocuous to noxious intensity of stimuli, whereas some fibers have high thresholds ( 40 mmHg) for response to esophageal distention (HTM-spinal, open square). Like LTM-spinal fibers, HT-spinal fibers exhibit increasing response to incrementing pressure. (Source: Sengupta et al.,59 with permission from the American Physiological Society.) b: Shows the frequency distribution of thresholds for response of vagal and spinal mechanosensitive afferent fibers. The majority of vagal afferent fibers exhibit response to distention <8 mmHg, whereas spinal afferents have two distinct populations. A greater number of fibers have low thresholds for response, and a relatively small number of fibers have high threshold for response.
40 mmHg) for response to esophageal distention (HTM-spinal, open square). Like LTM-spinal fibers, HT-spinal fibers exhibit increasing response to incrementing pressure. (Source: Sengupta et al.,59 with permission from the American Physiological Society.) b: Shows the frequency distribution of thresholds for response of vagal and spinal mechanosensitive afferent fibers. The majority of vagal afferent fibers exhibit response to distention <8 mmHg, whereas spinal afferents have two distinct populations. A greater number of fibers have low thresholds for response, and a relatively small number of fibers have high threshold for response.
Chemosensitivity of Muscle Tension-Sensitive Afferent Fibers
Muscle tension-sensitive afferent fibers may be sensitive to chemical stimuli. On average, 15% to 30% of muscle afferents in different species exhibit excitation to intraluminal acid (HCl) perfusion.61, 64, 71 (Figure 6).
Figure 6: Response of a vagal distention-sensitive afferent fiber to graded esophageal distention (ED) before and after acid infusion.
Fibers exhibited slowly adapting response to phasic ED. The receptive field of the fiber was located in the midthoracic esophagus. In each panel, the top trace represents firing frequency of the fiber and the bottom trace is ED pressure (5–80 mmHg). a: Response of the fiber to graded ED before acid infusion. b: Progressive increase in spontaneous firing of the fiber during and after acid infusion. The horizontal bar indicates the duration (4 minutes) of acid infusion. c: Response of the fiber to graded ED after acid infusion. imps, impulses. (Source: Medda et al.,71 with permission from Elsevier.)
It is a general notion that owing to their deep location in the muscle and the strong mucosal barrier, intraluminal acid is unable to excite the neurons.68 In studies in mice, a species that has a very thin musculature, mucosal acid fails to excite the muscle afferents.66 However, these muscle afferents easily respond chemical mediators such as  ,
, -methylene ATP and 5-hydroxytryptamine (5-HT), and agents such as bile and capsaicin, when applied on the mucosal surface.62, 64, 66 It is possible that these chemicals induce the release of some endogenous substances, which can excite the muscle afferents.
-methylene ATP and 5-hydroxytryptamine (5-HT), and agents such as bile and capsaicin, when applied on the mucosal surface.62, 64, 66 It is possible that these chemicals induce the release of some endogenous substances, which can excite the muscle afferents.
Studies have shown that muscle afferents are sensitive to the selective purinergic P2X3 agonist  ,
, -methylene ATP.44, 68 Interestingly, in the guinea pig, the drug exclusively activates nodose (embryologically derived from the placode) C-fibers, but not the jugular (embryologically derived from the neural crest) A- and C-fibers.68 In vitro, this drug is more effective when applied on the serosal surface, but fails to activate the neurons when applied on the mucosal surface.44, 68 The effect of
-methylene ATP.44, 68 Interestingly, in the guinea pig, the drug exclusively activates nodose (embryologically derived from the placode) C-fibers, but not the jugular (embryologically derived from the neural crest) A- and C-fibers.68 In vitro, this drug is more effective when applied on the serosal surface, but fails to activate the neurons when applied on the mucosal surface.44, 68 The effect of  ,
, -methylene ATP is very likely a direct activation of purinergic P2X2 and P2X3 receptors, because immunohistochemical studies have documented the presence of these receptors in the IGLEs.43, 46 Typically, P2X2 receptors are expressed in the small-, medium-, and large-diameter soma of the nodose ganglia, whereas P2X3 receptors are expressed in the small- and medium-diameter soma. All P2X2 and P2X3 positive somas are also isolectin-B4 (IB4) positive cells. As in DRGs, IB4-positive nodose somas do not contain substance P and CGRP.
-methylene ATP is very likely a direct activation of purinergic P2X2 and P2X3 receptors, because immunohistochemical studies have documented the presence of these receptors in the IGLEs.43, 46 Typically, P2X2 receptors are expressed in the small-, medium-, and large-diameter soma of the nodose ganglia, whereas P2X3 receptors are expressed in the small- and medium-diameter soma. All P2X2 and P2X3 positive somas are also isolectin-B4 (IB4) positive cells. As in DRGs, IB4-positive nodose somas do not contain substance P and CGRP.
Acid can excite primary sensory afferent fibers in the esophagus by activating two proton-gated channels: transient receptor potential vanilloid-1 (TRPV1) and acid-sensing ion channels (ASICs). The existence of these channels in the vagal and spinal afferents has recently been documented.72, 73, 74, 75
Interest in a possible role of the TRPV1 channel in acid-induced esophagitis is progressively building. Mice lacking TRPV1 channels develop significantly less esophagitis to acid exposure compared to wild-type mice.76 In addition, treating the wild-type mice with TRPV1 antagonist capsazepine can prevent the development of esophagitis. It has been documented that, in esophagitis, there is an over expression of TRPV1 in the esophagus.77
Transient receptor potential vanilloid-1 may contribute to esophagitis in two possible ways. First, activation of TRPV1 channels by acid can release neuropeptides (e.g., substance P and CGRP) from the nerve terminals of primary sensory afferents to induce plasma extravasations and inflammation. This is known as neurogenic inflammation. Second, once inflammation takes place, it induces the release of several inflammatory substances including the arachidonic acid derivative anandamide, lipoxygenase derivatives [e.g., 12-hydroperoxyeicosatetraenoic acid (12-HPETE), 15- hydroperoxyeicosatetraenoic acid (15- HEPTE) and leukotriene B4 (LTB4)] and long-chain unsaturated n-acyl dopamines. These substances are known as endovanilloids because they are potent agonists of TRPV1 channels.78, 79, 80 Therefore, activation of TRPV1 channels by these endovanilloids acts as a positive feedback loop to enhance the release of neuropeptides from the nerve terminals and other proinflammatory cytokines.
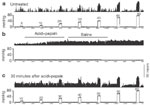
Capsaicin, a pungent derivative of hot chili pepper, can excite the muscle afferent fibers by activating the TRPV1 channels.81 This excitatory effect of capsaicin can be blocked by selective TRPV1 antagonist JYL1421. In chronic esophagitis in rats, muscle afferent fibers exhibit significantly greater response to capsaicin compared to noninflamed naive rats.81 This sensitized response to capsaicin is possibly due to an increase in the expression of TRPV1 channels in vagal afferent fibers that occurs after esophagitis (Figure 7).
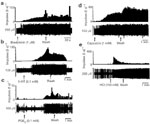
Figure 7: Transient receptor potential vanilloid-1 (TRPV1)–like immunoreactivity (TRPV1–IR) in rat nodose ganglia.
The low and high magnification images of TRPV1–IR are shown in naive control (a, d), acid-infused (b,e), and fundus-ligated (c,f). Most of the neurons in the fundus–ligated rats exhibit high levels of TRPV1–IR. The TRPV-ir in neurons from acid-infused rats varies from moderate to high intensity in comparison to a distinctly low TRPV1–IR in naive control. Scale bars in a-c 200  m; d-f 80
m; d-f 80  m.
m.
Acid-sensing ion channels are members of the amiloride-sensitive epithelial Na+/degenerin (eNac/DEG) channel superfamily. There are four subunits of ASIC: ASIC1, ASIC2, ASIC3, and ASIC4. Of these four subunits, ASIC1, ASIC2, and ASIC3 are present in primary sensory neurons. Although sensitivity of these channels to acid designated their name, it is still questionable whether acid is the natural ligand for these channels. These channels probably play more roles in mechanotransduction rather than sensing low pH. It has been shown that, in the vagus nerve, ASICs play complex roles in mechanotransduction.75, 82 In ASIC1a knockout mice, muscle afferent fibers exhibit increased sensitivity to mechanical stimulus, suggesting an inhibitory influence of this channel on mechanosensitivity. On the other hand, ASIC2 or ASIC3 knockout mice exhibit lesser mechanosensitivity.75
Roles of ASICs in acid-induced activation of muscle afferent fiber have not yet been studied systematically. The major difficulty is lack of selective ASIC blockers. Because amiloride and its analogues are nonselective sodium channel blockers and inhibit the overall excitability of vagal afferent fibers,82 it is difficult to distinguish the effect of acid on ASICs. Kollarik and Undem83 have shown that acid-induced activation of pulmonary A -fibers in the nodose ganglia is resistant to TRPV1 antagonist, which indirectly suggests an ASIC-mediated excitation by acid.
-fibers in the nodose ganglia is resistant to TRPV1 antagonist, which indirectly suggests an ASIC-mediated excitation by acid.
The tension-sensitive muscle afferents in the esophagus can be excited by bradykinin (BK), an algogenic substance.60, 64 Comparative study in opossum shows that, although systemic injection of bradykinin produces excitation of 66% of low threshold vagal afferent fibers, the excitation is primarily due to muscle contraction induced by BK. Paralysis of esophageal smooth muscle significantly attenuates the effect of BK.71 In contrast, spinal muscle afferents respond (100%) exquisitely to BK, and the excitation remains unaltered after muscle paralysis. Excitation of spinal afferents can be blocked by selective BK B2 receptor antagonists, but not by BK B1 receptor antagonists.60
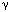 -Aminobutyric acid (GABA), an inhibitory neurotransmitter, activates two subtypes of receptors: GABA-A and GABA-B. A study has shown that the mechanotransduction property of the tension-sensitive vagal afferent fibers in ferrets can be attenuated by the GABA-B receptor agonist baclofen.84 Baclofen does not affect the spontaneous firing of the fibers, but at a dose
-Aminobutyric acid (GABA), an inhibitory neurotransmitter, activates two subtypes of receptors: GABA-A and GABA-B. A study has shown that the mechanotransduction property of the tension-sensitive vagal afferent fibers in ferrets can be attenuated by the GABA-B receptor agonist baclofen.84 Baclofen does not affect the spontaneous firing of the fibers, but at a dose  100
100  M it significantly inhibits responses of the fibers to muscle stretch. It is postulated that inhibition of the response of vagal sensory neurons by activating the peripheral GABA-B receptors at the receptive endings reduces the frequency of transient lower esophageal sphincter relaxation (TLESR).85
M it significantly inhibits responses of the fibers to muscle stretch. It is postulated that inhibition of the response of vagal sensory neurons by activating the peripheral GABA-B receptors at the receptive endings reduces the frequency of transient lower esophageal sphincter relaxation (TLESR).85
Galanin, a constitutive peptide of 30 amino acids, influences mechanosensitivity of the vagal tension-sensitive afferent fibers of ferrets and mice.86 Like GABA-B agonists, galanin (1–10 nM) predominantly inhibits responses of fibers to mechanical stimulus without much influence on spontaneous firing. However, a small subset of afferent fibers was excited by galanin. The effect of galanin appears to be via activation of galanin receptors as the galanin receptor antagonist galantide reverses the galanin effect. Although the exact functional role of galanin is not clear, it is speculated that galanin may regulate secretion and motility.86
Glutamate, the major excitatory neurotransmitter in the CNS, acts via the activation of ionotropic [N-methyl-D-aspartate (NMDA),  -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate] and metabotropic (mGlur group I, II, and III) receptors. Studies have shown that the glutamate receptors are not only restricted in the CNS, but are also present in the primary sensory neurons. The NMDA and AMPA receptors are present peripherally in the extrinsic vagal and spinal sensory afferent fibers87, 88, 89, 90, 91 innervating the GI tract. It has been shown that glutamate and NMDA receptors are involved in the regulation of TLESR.92 A recent study has documented that NMDA and AMPA receptor antagonists can effectively block the mechanotransduction properties of antral muscle afferent fibers.93 Similarly, NMDA antagonists attenuate mechanotransduction property of esophageal afferent fibers in rats (Figure 8).70
-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate] and metabotropic (mGlur group I, II, and III) receptors. Studies have shown that the glutamate receptors are not only restricted in the CNS, but are also present in the primary sensory neurons. The NMDA and AMPA receptors are present peripherally in the extrinsic vagal and spinal sensory afferent fibers87, 88, 89, 90, 91 innervating the GI tract. It has been shown that glutamate and NMDA receptors are involved in the regulation of TLESR.92 A recent study has documented that NMDA and AMPA receptor antagonists can effectively block the mechanotransduction properties of antral muscle afferent fibers.93 Similarly, NMDA antagonists attenuate mechanotransduction property of esophageal afferent fibers in rats (Figure 8).70
Figure 8: Effect of N-methyl-D-aspartate (NMDA) open channel blocker memantine hydrochloride on mechanotransduction property of an esophageal vagal afferent fiber innervating the esophagus of rat.
In each panel, the top trace represents firing frequency of the nerve fiber, the middle trace is the nerve action potentials, and the bottom trace is distending pressure (50 mmHg, 30 seconds). Memantine hydrochloride dose-dependently (1–100  mol/kg, i.v.) inhibited responses to esophageal distention.
mol/kg, i.v.) inhibited responses to esophageal distention.
Mucosal afferent endings are present below the mucosal epithelium. Most of the existing studies have been done in the vagus nerve. There is not much known about the mucosal mechanoreceptors in the spinal pathway. In general, mucosal afferents do not exhibit spontaneous activity and rapidly adapt to mechanical stimuli.94 Fibers are insensitive to peristaltic contractions and do not usually respond to intraluminal distention. These afferents are exquisitely sensitive to light touch on the mucosal surface and passage of a bolus through the lumen.29, 30, 61, 64, 67, 95, 96, 97, 98 In most of the species, these afferents exhibit a rapidly adapting response to mechanical probing of the mucosa. However, these afferents adapt slowly to chemical stimulus. In general, mucosal mechanoreceptors are found to be more sensitive to chemical stimuli than muscle tension-sensitive afferents, owing to the easier accessibility to the chemicals in the mucosa.
Sensitivity to Intraluminal Chemicals
Mucosal afferent fibers are relatively more sensitive to intraluminal application of chemicals. Figure 9 illustrates the response patterns of mucosal afferent fibers to different chemicals. Chemosensitivity of mucosal afferent fiber to intraluminal HCl is quite variable in different species. In the ferret, only 9% (3/33) mucosal afferents responded to topical application of 0.1 N HCl on the mucosa.64 Interestingly, acute esophagitis does not change the sensitivity of these afferents to HCl.65 In dogs and ferrets, HCl excites less than 10% of fibers. Repeated acid exposure desensitizes responses of mucosal afferents to subsequent acid and mechanical stimuli.64 In dogs and mice, mucosal afferents are not very sensitive to HCl. The mucosal barrier cannot fully explain the insensitivity of these afferents to acid, because mice, which have a relatively thin layer of mucosa and therefore more accessibility of mucosa afferents to chemical stimuli, show very little response to acid.66 Mucosal afferents have been shown to be insensitive to  ,
, -methylene ATP, a selective P2X receptor agonist, and it does not influence the mechanosensitivity of the fibers. However, after chronic esophagitis these afferents become significantly more sensitive to mechanical stimulus following
-methylene ATP, a selective P2X receptor agonist, and it does not influence the mechanosensitivity of the fibers. However, after chronic esophagitis these afferents become significantly more sensitive to mechanical stimulus following  ,
, -methylene ATP application.65
-methylene ATP application.65
Figure 9: Response characteristics of mucosal mechanosensitive afferent fibers.
Typical response characteristics of mucosal mechanosensitive afferent fibers of ferrets to bradykinin (a), serotonin (5-HT, b), prostaglandin E2 (PGE2, c), TRPV1 agonist capsaicin (d), and hydrochloric acid (HCl, e). Chemicals were applied topically on the mucosa after identification of the fiber's response to mechanical probing. In each panel, the top trace represents the firing frequency and the bottom trace is nerve action potentials. The responses to these chemicals are not from one mucosal afferent fiber. (Source: Page and Blackshaw,64 with permission.)
Unlike their response to HCl, mucosal afferents are more sensitive to bile, serotonin (5-HT), capsaicin, and prostaglandin.62, 64, 65, 66 It is not known, however, whether these drugs produce effects via activation of specific receptors. Effects of these chemicals have not been tested by blocking the receptors with a selective antagonist. Like muscle tension-sensitive afferent fibers, the mechanotransduction properties of the mucosal fibers can be significantly attenuated by the GABA-B agonist baclofen and galanin.75, 84 The physiologic roles of these drugs in the mucosa are not clearly known. However, it can be speculated that attenuation of response may reflexly regulate esophageal peristalsis.
Muscle Tension/Mucosal Afferents
The existence of a special type of afferent fibers has been reported in the esophagus of the ferret. These afferents respond to both mucosal stroking and to stretching of the circular muscle.64 Like mucosal afferents, a small proportion of these afferents are sensitive to chemicals, including capsaicin, HCl, 5-HT, bradykinin, and prostaglandin E2 (PGE2). Although morphologic study has not documented such endings, it is very likely that the axonal branching terminates in two different tissue layers.
Projection Spinal Afferents to the Spinal Cord
In general, the spinal cord is thought to be the pathway for nociceptive (i.e., painful sensation) transmission. Studies in humans have documented that prolonged acid perfusion produces hypersensitivity of the esophagus to distention owing to spinal sensitization.14, 15 Although spinal afferents from the esophagus enter the spinal cord at every segment of the cervical and thoracic spinal cord, the greatest number of projections is found in the upper cervical (C1–C4) and thoracic (T3–T5) segments. The majority (>65%) of the spinal dorsal horn neurons that receive projection from the spinal afferents also receive projection from the skin and muscle of the trigeminal area, neck, shoulder, chest, and retrosternal area. Almost 25% of the ED-sensitive neurons also receive convergent input from the heart.7, 9, 99, 100 The ED-sensitive spinal neurons can be excited by injection of bradykinin into the pericardial sac.7, 99 This convergence of cardiac and esophageal afferents in the spinal neurons is the main cause of the confusion of angina-type pain referred to the retrosternal area.
Response Characteristics of Spinal Neurons
Extracellular recordings in rats have identified four types of spinal neurons according to their response patterns to phasic ED99, 100: (1) tonic excitatory neurons without poststimulus firing, (2) tonic excitatory neurons with poststimulus firing, (3) inhibited neurons, and (4) initially excited followed by inhibition of firing (Figure 10). Of four classes of neurons, 85% of these neurons exhibit excitation, 12% show inhibition, and 3% show excitation followed by inhibition.
Figure 10: Responses of a distention-responsive upper cervical (C2) spinal neuron having convergent inputs from the cervical and thoracic esophagus.
a,b: The intensity-dependent increase in responses to distention of cervical and thoracic esophagus, respectively, in the intact condition. c,d: The responses after ipsilateral (i.e., on the recording site) cervical vagotomy. There is reduction in response to thoracic distention after ipsilateral vagotomy (c). e,f: The responses following bilateral vagotomy. There is a significant reduction in response to thoracic distention compared to cervical distention after bilateral vagotomy. g,h: The neuron stopped responding to both cervical and thoracic distention after spinal transection at C6 level. (Source: Qin et al.,101 with permission from the American Physiological Society.)
In rats, the cervical (C1–C2) spinal cord also receives convergent input from the cervical and thoracic esophagus.101 It has been shown that distention of the cervical and thoracic segments of the esophagus can produce responses of spinal neurons in the upper cervical (C1–C2) spinal cord, indicating that upper cervical neurons receive convergent input from two different areas of the esophagus. Interestingly, responses of these neurons are dependent on neural connectivity. For example, responses to cervical ED remain unchanged when bilateral cervical vagi and the lower (i.e., below the recording site) cervical (C6–C7) spinal cord is transected, indicating that that these neurons receive input from the upper cervical spinal afferent fibers. However, responses of 50% neurons to thoracic ED are abolished after bilateral cervical vagotomy and 100% following C6–C7 spinal transaction (Figure 10). Thus, it clearly indicates that input from the thoracic esophagus is partly dependent on vagal pathways as well as the spinal afferent pathways entering via the thoracic spinal cord.101 It can be concluded from this study that projections of spinal afferents from the different region of the esophagus do not strictly follow the segmental distribution and can activate many segments of the spinal cord. This overlapping of spinal projections may explain the secondary hyperalgesia in the upper esophagus of the human volunteers that was observed when acid was infused into the distal part of the esophagus.14
Sensitization of Spinal Neurons
In cats, when the esophagus is inflamed with turpentine, the thresholds for response of ED-sensitive spinal neurons significantly decrease compared to saline-treated cats.7 Thus, this result indicates that following noxious stimulus in the esophagus, sensitization of spinal neurons takes place, which can explain the esophageal hypersensitivity observed after prolonged acid infusion.14, 15, 17, 18, 19, 20, 21 It should also be noted that sensitization is not exclusively dependent on chemical stimuli. In experimental animals, it has been shown that repeated noxious distention can produce progressive sensitization of response of the spinal neurons to subsequent ED as well as an expansion of the convergent somatic receptive field.8, 99
Projection to the Brainstem Neurons
Brainstem nuclei play coordinating roles in supraesophageal reflexes, deglutition, secondary peristalsis, belching, and gastroesophageal reflexes.102, 103, 104 Esophageal vagal afferents mostly terminate in the central subnucleus of the nucleus tractus solitarius (NTS).105, 106, 107 The second-order neurons from the NTS mainly project to two motor nuclei including the nucleus ambiguus (NA) and the dorsal motor nucleus of the vagus (DMV). Anatomic and physiologic studies have revealed that rodents, with an esophagus predominantly composed of skeletal muscle, have preganglionic motor neurons mostly located in the NA. However, in humans and cats, which have the lower one third of the esophagus composed of smooth muscle, preganglionic motor neurons arise from DMV.35, 106, 108, 109 In the striated esophagus, NTS neurons intricately regulate the deglutitive reflexes.
Types of Nucleus Tractus Solitarius Neurons Receiving Vagal Input
Extracellular recordings from the central nucleus of the NTS (NTSc) of rats have identified three types of neurons that respond differently to ED and peristalsis (Figure 11).110 Type I neurons show a rhythmic burst of discharge during distal ED, which is in phase with peak intraluminal pressure, but exhibit inhibition to midthoracic distention. During distal ED and rhythmic contraction of the distal esophagus, midthoracic distention produces inhibition of rhythmic contractions of the distal esophagus, as well as decrease in firing of the type I neurons, suggesting that these neurons receive excitatory inputs from the distal esophagus that cease firing during inhibition of contraction. Type II neurons exhibit a tonic firing without any rhythmic pattern during peristaltic contraction. Unlike type I neurons, these neurons are excited during distention of both the distal and midthoracic esophagus, and firings do not decrease when distal peristalsis is inhibited during midthoracic distention. This suggests that type II neurons receive excitatory input from a wide area of the esophagus. Type III neurons are either partially or completely inhibited by distention, but show a rebound firing after the termination of distention. Although the specific functions of these three types of neurons have not been clearly delineated, it is speculated that they take part in the deglutitive reflex.
Figure 11: Typical firing patterns of three types of neurons in the nucleus tractus solitarius centralis (NTSc) region receiving synaptic input from the vagal afferent fibers.
a: The type I neuron that exhibits rhythmic burst of firing in phase with intraluminal pressure change during the distention of the distal esophagus (DE). However, the neuron stopped firing during the distention of the thoracic esophagus (TE). The enhanced firing and intraluminal pressure during DE distention can be attenuated by TE distention. b: Unlike the type I neuron, the type II neuron exhibits nonrhythmic excitation during TE or DE distention. Firing of this neuron does not attenuate during the inhibition of intraluminal pressure produced by TE distention. c: The type III neuron fires during the falling phase of the esophageal pressure wave during DE distention. The neuron stops firing to TE distention alone or in combination with DE distention. (Source: Dong et al.,110 with permission from American Gastroenterological Association.)
Sensitization of the Nucleus Tractus Solitarius Neurons
Like spinal neurons, sensitization of NTS neurons may lead to esophageal hypersensitivity. It has been reported that acid infusion into the distal esophagus activates 56% of vagal motor neurons that respond to ED.111 It is conceivable that excitation of vagal motor neurons may occur due to excitatory synaptic projection from the NTS neurons. However, a direct activation of DMV by vagal afferent fibers cannot be excluded, because anatomic study has demonstrated that a proportion of vagal afferent fibers directly project onto the DMV.112 A recent study demonstrates that an acute intraesophageal acid and pepsin infusion enhances baseline spontaneous firing of NTS neurons and sensitizes responses to ED.69 It appears that this sensitization does not require a sustained low pH in the esophagus, because pH monitoring studies clearly indicate that the decline in pH owing to acid infusion can be recovered by saline wash in a relatively short period of time (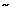 10 minutes). However, the enhanced firing of brainstem neurons outlasts the neutralization of the low pH in the esophagus. It also appears that although the excitation of these neurons requires excitation of vagal afferent fibers, their sensitized response to ED does not require persistent sensitization of vagal afferent fibers to ED.71 It is to be noted that distention-sensitive vagal afferents do not exhibit a sensitized response to ED, but demonstrates a persistently high spontaneous firing rate after acid exposure (Figure 2). However, this observation cannot rule out the contribution of other vagal afferent fibers (i.e., mucosal afferent fibers). It is very likely that NTS neurons receive multiple converging projections from other vagal sensory afferents, including mucosal afferents that may contribute to the sensitization of their response. In either case, it is reasonable to think that frequent acid exposure into the esophagus may lead to sensitization of brainstem neurons that influence the preganglionic motor neurons in the DMV and NA, resulting in esophageal hypersensitivity/hypermotility.
10 minutes). However, the enhanced firing of brainstem neurons outlasts the neutralization of the low pH in the esophagus. It also appears that although the excitation of these neurons requires excitation of vagal afferent fibers, their sensitized response to ED does not require persistent sensitization of vagal afferent fibers to ED.71 It is to be noted that distention-sensitive vagal afferents do not exhibit a sensitized response to ED, but demonstrates a persistently high spontaneous firing rate after acid exposure (Figure 2). However, this observation cannot rule out the contribution of other vagal afferent fibers (i.e., mucosal afferent fibers). It is very likely that NTS neurons receive multiple converging projections from other vagal sensory afferents, including mucosal afferents that may contribute to the sensitization of their response. In either case, it is reasonable to think that frequent acid exposure into the esophagus may lead to sensitization of brainstem neurons that influence the preganglionic motor neurons in the DMV and NA, resulting in esophageal hypersensitivity/hypermotility.
Conclusion and Speculations for the Future
Esophageal hyperalgesia can occur owing to hyperexcitability of peripheral sensory neurons innervating the esophagus (peripheral sensitization) and heightened response of neurons in the spinal cord and brain (a central sensitization). It is well recognized from the clinical observations and reports from psychophysical studies that frequent acid exposure can lead to esophagitis, and heavy acid burden often produces esophageal hypersensitivity. Although healing of erosive esophagitis can be controlled by the use of proton pump inhibitors (PPIs), therapy for nonerosive reflux disease (NERD) and NCCP still remains challenging. Acid primarily activates two proto-gated ion channels (TRPV1 and ASICs) that are present in the sensory neurons. Activation of these channels results in neurogenic inflammation as well as sensitization of sensory neurons. It is also possible that acid can induce the release of proinflammatory substances from the nonneuronal tissues that sensitize sensory neurons and ultimately initiate central sensitization. Therefore, selective TRPV1 or ASICs antagonists that can potentially block the acid-induced tissue injury may prove therapeutically important to prevent esophageal hypersensitivity and tissue inflammation. Currently, the most common therapy for GERD is the use of a PPI to decrease the gastric acid production. However, it does not alleviate the pain and discomfort of chronic GERD or NCCP that can be due to central spinal sensitization. Therefore, in the case of chronic esophageal hyperalgesia, selective and potent blockers targeted to substance P receptors [neurokinin 1 (NK1, 2 (NK2), and 3 (NK3)] and glutamate receptors (NMDA, AMPA, and metabotropic glutamate receptor) may turn out to be effective therapy.



![Figure 4 : Intensity-dependent response of a vagal afferent fiber to graded esophageal distention (ED, 5|[ndash]|80 mmHg, 30 seconds). Unfortunately we are unable to provide accessible alternative text for this. If you require assistance to access this image, or to obtain a text description, please contact npg@nature.com](/gimo/contents/pt1/thumbs/gimo16-f4.jpg)

![Figure 7 : Transient receptor potential vanilloid-1 (TRPV1)|[ndash]|like immunoreactivity (TRPV1|[ndash]|IR) in rat nodose ganglia. Unfortunately we are unable to provide accessible alternative text for this. If you require assistance to access this image, or to obtain a text description, please contact npg@nature.com](/gimo/contents/pt1/thumbs/gimo16-f7.jpg)