Abstract
Purpose:
The goal of this study was to examine participant responses to disclosure of genetic results in a minority population at high risk for depression and anxiety.
Methods:
Eighty-two subjects in a genetic study of nicotine dependence were offered personalized genetic results. All were nicotine-dependent and 64% self-identified as African American. Pathway Genomics was used to evaluate genetic risks for five complex diseases. Participants returned 4–8 weeks after enrollment for in-person genetic counseling interviews and evaluation of baseline measures. A telephone follow-up was performed 4–8 weeks later to assess responses to results.
Results:
Fifty of the 82 subjects (61%) were interested in receiving genetic results. These participants had multiple risk factors, including high baseline measures of depression (66%) and anxiety (32%), as well as low rates of employment (46%), adequate health literacy (46%), and health insurance (45%). Pathway Genomics reported “increased risk” for at least one disease in 77% of subjects. Ninety-five percent of participants reported that they appreciated the genetic results, and receiving these results was not associated with changes in symptoms of depression or anxiety. Furthermore, after return of genetic results, smoking cessation attempts increased (P = 0.003).
Conclusion:
Even in an underserved population at high risk for adverse psychological reactions, subjects responded positively to personalized genetic results.
Genet Med 17 5, 374–379.
Similar content being viewed by others
Introduction
Over the past decade, the return of individual genetic research results has emerged as a highly contentious issue.1,2,3,4,5 Advocates assert that researchers and clinicians have an ethical obligation to offer certain results when possible, based on principles of beneficence and respect of persons.1,2 Critics emphasize possible harmful effects, including therapeutic misconceptions, financial burdens, and undue anxieties.1,3,4 Similar concerns regarding potential downstream consequences of individual genetic results have been raised in consumer settings with the increased use of personalized genetic testing, especially for direct-to-consumer companies.6,7,8,9,10 As genomics research marches forward, a better understanding of how individuals respond to personalized genetic results is critical for informing future policies and decisions regarding appropriate use of this information.
There has been recent controversy over direct-to-consumer genetic testing. Specifically, the US Food and Drug Administration issued concerns that 23andMe was inappropriately offering genetics-based medical recommendations.11 Because of this, 23andMe stopped offering medical reports with the genetic testing. Rather, 23andMe offers the same genetic testing and ancestry report, but no medical reports.
Despite the controversy concerning 23andMe, studies demonstrate that the public strongly favors opportunities for disclosure of individual genetic results.12,13,14,15,16,17 Although African Americans are often underrepresented in genetics research, focus groups indicate that many individuals are interested in receiving their genetic results,12,14 and offering results may encourage future research participation.14 In response to the public’s interest in individualized genetic information, a growing number of studies over the past 5 years have examined subject responses to actually receiving genetic findings.18,19,20,21,22,23 These studies suggest that return of genetic results does not pose substantial psychological or behavioral harms. Because these studies focused primarily on populations that are at relatively low risk for adverse psychological reactions (the majority of subjects studied are college-educated European Americans with health insurance who are employed or retired), the generalizability of these positive findings remains unclear.
An important next step is to investigate participant responses to personalized genetic results in a population at high risk for adverse psychological reactions. In this study, individual research results regarding complex disease risks were returned to participants in a genetic study of nicotine dependence: the majority of participants were African American, not college educated, unemployed, and uninsured. These data provide critical insight into the psychological and behavioral impact of personalized genetic results in a population that has traditionally been severely underrepresented in genetics research.
Materials and Methods
Setting and participants
This return of results study builds on an ongoing genetic study of nicotine dependence, the Collaborative Genetic Study of Nicotine Dependence (COGEND).24,25 In 2013, COGEND was recruiting nicotine-dependent smokers from the St. Louis metropolitan area. All nicotine-dependent COGEND subjects were eligible for this return of results study until 50 subjects were recruited. After completion of the COGEND interview, eligible subjects interested in participating in this return of results study were read basic explanations of the genetic testing procedures and the interpretation of genetic results. Those who expressed continued interest were given a complete description of the study design, including example genetic results (Supplementary Material online). Individuals who chose to be study participants then signed an informed consent form. This study protocol was approved by the institutional review board at Washington University School of Medicine.
Study design
Participants were assessed three times: at the time of consent, before receiving genetic results (4–8 weeks after consent), and during a telephone follow-up (4–8 weeks after return of genetic results and genetic counseling). At the time of consent, participants were assessed for anxiety using the Beck Anxiety Inventory26 and for depressive symptoms using the Center for Epidemiological Studies Depression Scale.27 Participants donated saliva samples, which were sent to Pathway Genomics (San Diego, CA) for microarray-based genetic analysis of risk for five complex diseases: lung cancer, breast or prostate cancer, colorectal cancer, heart attack, and type II diabetes (example results are given in the Supplementary Material online). Pathway Genomics genotyped the saliva samples for known genetic variants related to these diseases and used a proprietary algorithm to define individuals as being at “increased risk,” “above-average risk,” and “average risk.”
Pathway Genomics was chosen for this return of results study because it is accredited in accordance with the US Health and Human Services Clinical Laboratory Improvement Amendments of 1988 and therefore meets basic standards for return of results. Genotyping from the original parent study of nicotine dependence was not performed in a Clinical Laboratory Improvement Amendments–certified laboratory. We chose to return genetic results of five complex diseases for which smoking cessation is critical for reducing overall risk. Of note, the strongest genetic risk factor for lung cancer is the same as the strongest genetic risk for smoking behavior. Pathway Genomics does not offer reports on nicotine addiction.
Participants returned 4–8 weeks after donating samples to receive their genetic results. At the beginning of this meeting, each participant was assessed for health literacy using the REALM-R.28 Current health-care accessibility, smoking behavior, cessation attempts, diet, and exercise were also assessed.
Subsequently, the results were given to the participant in the context of a genetic counseling session. Although genetic counselors were given up to 1 hour to meet with each subject, the average counseling session lasted ~10 min. The genetic counselor gave personalized results to each participant and emphasized the importance of smoking cessation. The counselor then offered to answer any questions the subjects had about their results. Before leaving, subjects were given a folder containing individual genetic results (without identifying information) and smoking cessation tips.
Each participant was called on the telephone 4–8 weeks after receiving the genetic results and asked about his or her response to the results, as well as current health-care accessibility, smoking behavior, diet, exercise, symptoms of depression (Center for Epidemiological Studies Depression Scale),27 and symptoms of anxiety (Beck Anxiety Inventory).26
Statistical analysis
All statistical analyses were performed using Statistical Analysis System (SAS 9.3; SAS Institute, Cary, NC). Changes in dichotomous variables over time (depression, anxiety, and smoking cessation attempts in the past month) were evaluated using McNemar’s test, and changes in continuous variables (change in depression and anxiety scales) were modeled using linear regression. Power calculations (SAS PROC POWER) were also used.
Results
Participant characteristics
Eighty-two subjects were offered return of genetic results; 61% (50/82) of these subjects accepted the invitation to participate ( Figure 1 ). Of the 32 subjects who declined participation, 41% (13/32) reported scheduling conflicts. No statistical differences between the participants (n = 50) and nonparticipants (n = 32) (P > 0.05) for sex, race, educational attainment, employment status, or age (details of demographics in Table 1 ) were observed.
The participants themselves were at very high risk for psychiatric events ( Table 1 ). Specifically, their baseline symptoms of depression and anxiety (strong predictors of future major depression disorder and anxiety)29 were very high. In addition, many subjects had limited health literacy, had low education levels, were unemployed, or were uninsured ( Table 1 ). These factors increase the chance of confusion or distress, which may in turn increase the risk for depression or anxiety.
Genetic results
The participants reported an overwhelmingly positive reaction to the results: 95% of the subjects said they found the results worthwhile. All but one subject discussed the results with someone outside the study, including their doctors (17%) and family or friends (95%).
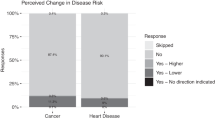
Most of the subjects (77%; 33/43) received a genetic report with “increased risk” for at least one of the five tested diseases. The most common disease with increased risk was colorectal cancer (16/43). None of the women received a report stating they had an increased risk for breast cancer (BRCA1 and BRCA2 variants were not genotyped; Supplementary Material online).
Two subjects reported being upset by the results. Both of these subjects had “above average” risk for lung cancer; one also had “above average” risk for heart disease. Both found the results worthwhile, both told their friends about the results, and neither had a clinically significant increase in anxiety.
Changes in depression and anxiety
Receiving genetic results did not lead to changes in the proportion of subjects meeting criteria for depression as defined by a Center for Epidemiological Studies Depression Scale score ≥16, or anxiety as defined by a Beck Anxiety Inventory score ≥16 ( Table 2 ). In addition, even for individuals who received reports indicating increased risk of any disease or each specific disease, we observed no significant increase in the overall proportion of subjects with depression or anxiety ( Table 2 ).
To further investigate the impact of receiving genetic results on symptoms of depression and anxiety, we evaluated whether increased risk of disease was related to change in depression or anxiety scores ( Table 3 ). No associations were seen between changes in depression or anxiety scores and reported genetic risk of disease ( Table 3 ).
A power analysis was used to determine whether lack of a statistical association suggests lack of a clinical association. A clinically significant increase in symptoms of depression was defined as an increase of 6 on the Center for Epidemiological Studies Depression Scale score, which corresponds to two symptoms of depression increasing from “rarely” to “most or all of the time.” Similarly, a clinically significant increase in symptoms of anxiety was defined as an increase of 6 on the Beck Anxiety Inventory score, which corresponds to anxiety increasing from “no anxiety” to “mild anxiety,” or “mild anxiety” increasing to “moderate anxiety,” Using the observed sample size and SE, we calculated that there was 89% power to detect a clinically significant change in depression and 97% power to detect a clinically significant change in anxiety. This suggests that we had adequate power to detect clinically significant effects in the overall sample.
Behavioral response
Our secondary analyses examined whether return of genetic results changed behavior. Attempts at smoking cessation were measured at two time points: baseline of 4–8 weeks after the parent genetic study of nicotine dependence (at the time of genetic counseling) and follow-up of 4–8 weeks after genetic counseling for the return of results study ( Table 4 ). Subjects were more likely to attempt smoking cessation after receiving genetic results relative to a similar duration of time after participation in the study of nicotine dependence alone (P = 0.003). A strong increase in attempts to quit occurred among individuals who received reports with increased genetic risk for lung cancer (quit attempts increased from 14% to 57%), but this difference was not statistically significant (P = 0.08).
Discussion
After receiving personalized genetic results, participants from a genetic study of nicotine dependence reported that the results were useful and they did not experience an increase in symptoms of depression or anxiety. This overall positive response to return of genetic results is particularly noteworthy because the sample is comprised of underserved individuals at high risk for adverse psychological events, as evidenced by high rates of baseline symptoms of depression and anxiety, as well as low rates of employment, health insurance, and health literacy.
Overall, this study had a high participation rate (61%) and almost all of the participants indicated that receiving genetic results was worthwhile (95%), suggesting that individuals from minority high-risk populations appreciate personalized genetic findings. This extends results from focus groups that found that the majority of African Americans were interested in receiving genetic results.12,14
Participants did not have increased scores on depression or anxiety measures in response to receiving genetic risk information on complex diseases, results similar to those of studies of other populations at relatively lower risk for adverse events.18,22 Bloss et al.18 offered subsidized personalized genetic testing of complex diseases to individuals working in health and technology, and responses from more than 2,000 participants showed no measurable changes in anxiety 3 months after receiving genetic information. In addition, preliminary findings from a survey of 1,800 customers of two genomics companies suggest there is no elevation in anxiety or distress during the year after receiving genetic results.22 Our findings build on these studies by demonstrating that even in a high-risk population defined by low health-care literacy, high levels of unemployment, and lack of insurance, symptoms of depression and anxiety were not increased after disclosure of genetic complex disease information.
Beyond not causing a substantial increase in symptoms of depression or anxiety, after disclosure of genetic risks for complex diseases there was an increase in smoking cessation attempts. This raises the question of whether return of genetics results may motivate smoking cessation attempts in a nicotine-dependent population, contributing to the debate over whether genetic information can motivate risk-reducing health behaviors.30 Specifically, subjects were significantly more likely to make quit attempts 4–8 weeks after return of results in the genetic counseling session as compared with a baseline measured 4–8 weeks after the original interview for the parent study of nicotine dependence. This finding suggests that the smoking behavioral change was driven by the process of receiving personalized genetic results and not simply by interviewer contact or discussion about smoking cessation. However, further studies that include a control group are necessary to determine whether increased quit attempts are attributable to receiving personalized genetic results rather than confounding factors such as increased study engagement.
Even though we observed an increase in attempts to quit smoking, cessation is difficult, and often numerous attempts are made before smokers are able to successfully quit. A recent meta-analysis found that communication of DNA-based risk estimates was not associated with smoking cessation.31 However, we can speculate that increased motivation to quit during the period after return of genetic results may be an opportune time for intensive smoking cessation therapies.
This study has several limitations. First, we used a convenience sample recruited through a parent study of nicotine dependence, and our study did not include a control group. Second, the sample size of 50 participants is modest; therefore, the observed results may be driven by a limited number of individuals. Nonetheless, the study was appropriately powered to detect clinically significant changes in symptoms of depression and anxiety (89 and 97%, respectively). Third, we returned genetic risk results of five complex diseases and did not include highly penetrant genetic variants, such as BRCA1 or BRCA2 mutations. It is possible that results associated with a greater increased risk of disease may prompt additional distress. Studies that returned pathogenic variants in APOE for Alzheimer disease,20 BRCA1 and BRCA2 for hereditary breast and ovarian cancer,19 and CDKN2A for melanoma23 found that these potentially alarming disclosures caused only modest distress. Additional studies are necessary to extend these analyses to underserved and high-risk populations. Fourth, our findings are based on a single follow-up assessment at 1–2 months and cannot be used to assess the long-term effects of the genetic results. In addition, it would be useful to expand the qualitative component of this study. Future qualitative and quantitative studies that follow research participants after receiving genetic results are needed to better understand their perceptions of genetic risk and how it shapes health outcomes over the long term.
Conclusion
This study lays the foundation for understanding how high-risk underserved populations respond to personalized genetic results, informing the debate surrounding the consequences of returning these results. Specifically, we demonstrate that, similar to other populations, high-risk minority participants appreciate return of results, do not have increased symptoms of depression or anxiety, and may benefit from receiving individual genetic information. Technical and scientific barriers regarding the disclosure of genetic results in research, clinical, and consumer settings still exist, and overcoming them will require continued progress in several areas, such as definitive identification and characterization of genetic risk factors, validation of algorithms to estimate risk, and development of standardized policies and procedures to prioritize and communicate genetic information. The empirical evidence presented in this study suggests that once these barriers are crossed, personalized genetic results may be positively received in underserved populations.
Disclosure
L.B. is listed as an inventor on Issued U.S. Patent 8,080,371,“Markers for Addiction,” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. D.K. is an unpaid consultant for an ongoing academic study of customers of 23andMe and Pathway Genomics. The other authors declare no conflict of interest.
References
Bredenoord AL, Kroes HY, Cuppen E, Parker M, van Delden JJ . Disclosure of individual genetic data to research participants: the debate reconsidered. Trends Genet 2011;27:41–47.
Manolio TA . Taking our obligations to research participants seriously: disclosing individual results of genetic research. Am J Bioeth 2006;6:32–34; author reply W10.
Meltzer LA . Undesirable implications of disclosing individual genetic results to research participants. Am J Bioeth 2006;6:28–30; author reply W10.
Clayton EW, Ross LF . Implications of disclosing individual results of clinical research. JAMA 2006;295:37; author reply 37–37; author reply 38.
Wolf SM, Lawrenz FP, Nelson CA, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics 2008;36:219–48, 211.
Evans JP, Green RC . Direct to consumer genetic testing: avoiding a culture war. Genet Med 2009;11:568–569.
Caulfield T . Direct-to-consumer genetics and health policy: a worst-case scenario? Am J Bioeth 2009;9:48–50.
Kuehn BM . Risks and benefits of direct-to-consumer genetic testing remain unclear. JAMA 2008;300:1503–1505.
McGuire AL, Burke W . An unwelcome side effect of direct-to-consumer personal genome testing: raiding the medical commons. JAMA 2008;300:2669–2671.
Sanderson SC, Humphries SE, Hubbart C, Hughes E, Jarvis MJ, Wardle J . Psychological and behavioural impact of genetic testing smokers for lung cancer risk: a phase II exploratory trial. J Health Psychol 2008;13:481–494.
Gutierrez A . Warning Letter. 2013. http://www.fda.gov/iceci/enforcementactions/warningletters/2013/ucm376296.htm. Accessed 15 December 2013.
Yu JH, Crouch J, Jamal SM, Tabor HK, Bamshad MJ . Attitudes of African Americans toward return of results from exome and whole genome sequencing. Am J Med Genet A 2013;161A:1064–1072.
Bollinger JM, Scott J, Dvoskin R, Kaufman D . Public preferences regarding the return of individual genetic research results: findings from a qualitative focus group study. Genet Med 2012;14:451–457.
O’Daniel J, Haga SB . Public perspectives on returning genetics and genomics research results. Public Health Genomics 2011;14:346–355.
Meulenkamp TM, Gevers SK, Bovenberg JA, Koppelman GH, van Hylckama Vlieg A, Smets EM . Communication of biobanks’ research results: what do (potential) participants want? Am J Med Genet A 2010;152A:2482–2492.
Kaufman D, Murphy J, Scott J, Hudson K . Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med 2008;10:831–839.
Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K . Public expectations for return of results from large-cohort genetic research. Am J Bioeth 2008;8:36–43.
Bloss CS, Schork NJ, Topol EJ . Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med 2011;364:524–534.
Francke U, Dijamco C, Kiefer AK, et al. Dealing with the unexpected: consumer responses to direct-access BRCA mutation testing. PeerJ 2013;1:e8.
Green RC, Roberts JS, Cupples LA, et al.; REVEAL Study Group. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med 2009;361:245–254.
Kaphingst KA, McBride CM, Wade C, et al. Patients’ understanding of and responses to multiplex genetic susceptibility test results. Genet Med 2012;14:681–687.
Green RC, Farahany NA . Regulation: The FDA is overcautious on consumer genomics. Nature 2014;505:286–287.
Christensen KD, Roberts JS, Shalowitz DI, et al. Disclosing individual CDKN2A research results to melanoma survivors: interest, impact, and demands on researchers. Cancer Epidemiol Biomarkers Prev 2011;20:522–529.
Saccone NL, Culverhouse RC, Schwantes-An TH, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet 2010;68:e1001053.
Saccone NL, Schwantes-An TH, Wang JC, et al. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav 2010;9:741–750.
Beck AT, Epstein N, Brown G, Steer RA . An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893–897.
Radloff L . The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401.
Bass PF 3rd, Wilson JF, Griffith CH . A shortened instrument for literacy screening. J Gen Intern Med 2003;18:1036–1038.
Van Voorhees BW, Paunesku D, Gollan J, Kuwabara S, Reinecke M, Basu A . Predicting future risk of depressive episode in adolescents: the Chicago Adolescent Depression Risk Assessment (CADRA). Ann Fam Med 2008;6:503–511.
McBride CM, Koehly LM, Sanderson SC, Kaphingst KA . The behavioral response to personalized genetic information: will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health 2010;31:89–103.
Marteau TM, French DP, Griffin SJ, et al. Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database Syst Rev 2010(10):Cd007275.
Acknowledgements
This work was supported by National Institutes of Health grants K08DA032680, P01CA089392, R01CA168608, R01DA025888, R21DA033827, T32GM07200, TL1TR000449, UL1TR000448, UL1RR024992, U01HG005217, and 3U54CA153460-03S1.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Material
(PDF 678 kb)
Rights and permissions
About this article
Cite this article
Hartz, S., Olfson, E., Culverhouse, R. et al. Return of individual genetic results in a high-risk sample: enthusiasm and positive behavioral change. Genet Med 17, 374–379 (2015). https://doi.org/10.1038/gim.2014.110
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2014.110
Keywords
This article is cited by
-
Behavioral and psychological impact of genome sequencing: a pilot randomized trial of primary care and cardiology patients
npj Genomic Medicine (2021)
-
Most Current Smokers Desire Genetic Susceptibility Testing and Genetically-Efficacious Medication
Journal of Neuroimmune Pharmacology (2018)