Abstract
Purpose:
The aim of this article is to describe cancer risk–reducing behaviors of women with BRCA variants of unknown significance.
Methods:
A retrospective chart review from 1995 to 2012 identified women with BRCA mutations in a northern California community system. Exclusion criteria included loss of membership/death within 1 year of testing, prior ovarian cancer, or bilateral salpingo-oophorectomy. Primary outcomes were rate of risk-reducing mastectomy and risk-reducing salpingo-oophorectomy.
Results:
The mean age of the 69 variant of unknown significance carriers was 50 vs. 47 years for the 305 women with a deleterious mutation. Women with a variant of unknown significance were followed for a median of 69 months. Among women with a variant of unknown significance, 30% underwent risk-reducing salpingo-oophorectomy and 11% underwent risk-reducing mastectomy, as compared with 74 and 44%, respectively, for women with a deleterious mutation. Women with a deleterious mutation were more likely to undergo surveillance in the first year after testing. The odds ratios are as follows: 2.1 for mammogram, 6.0 for magnetic resonance imaging, 7.7 for Ca-125, and 5.0 for transvaginal ultrasound. Fifty-six percent of women with a variant of unknown significance were reclassified after a median of 39 months, longer than the median time to risk-reducing salpingo-oophorectomy (18.6 months) or risk-reducing mastectomy (20.1 months).
Conclusion:
Uptake of risk-reducing strategies among women with a variant of unknown significance is lower than among women with a deleterious mutation. Given the prognostic uncertainty and high rate of reclassification for women with a variant of unknown significance, individualizing counseling and directing efforts toward surveillance, chemoprevention, or salpingectomy are recommended.
Genet Med 16 12, 896–902.
Similar content being viewed by others
Introduction
BRCA1 and BRCA2 carriers have a 65–74% lifetime risk of breast cancer and a 39–46% and 12–20% respective lifetime risk of ovarian cancer.1 National evidence-based guidelines recommend strategies for these women to reduce their risk of cancer and lower their cancer-related mortality. However, ~7–15% of women tested for BRCA are positive for a variant of unknown significance (VUS), with greater percentages of African-American and Hispanic women testing positive for this type of mutation at rates of 21–45% and 36%, respectively.2,3 At the time of testing, the result in these women may be classified as “probable benign polymorphism,” “suspect deleterious,” or “truly unknown variant.” These women are faced with uncertainty regarding their cancer risk and what actions are appropriate to reduce that risk. Many of these variants are reclassified over time, prolonging the period of uncertainty. Management and counseling of this group can be challenging because it is difficult to predict their cancer risk, and no guidelines have been developed for recommending risk-reducing strategies in these women.
The National Comprehensive Cancer Network has developed a set of surveillance guidelines and surgical risk-reduction recommendations aimed at decreasing disease in women with known deleterious BRCA mutations. Risk-reducing salpingo-oophorectomy (RRSO) is recommended by the age of 40 years or after childbearing, and risk-reducing mastectomy (RRM) should be discussed as an option for all women with hereditary breast and ovarian cancer syndrome.4 Undergoing these procedures can dramatically reduce the risk in BRCA carriers, with RRM decreasing risk of breast cancer by 90% and RRSO decreasing ovarian cancer by 85–90% and breast cancer by 50–60% (ref. 1). In the United States, it is estimated that 20–49% of BRCA carriers undergo RRM and 37–60% undergo RRSO.5,6,7,8,9
In contrast to surgical intervention, more conservative chemopreventive measures and surveillance practices may also be beneficial for women at higher risk of developing breast and ovarian cancers. Oral contraceptive use can reduce risk of ovarian cancer by 50–60%, and tamoxifen can reduce risk of breast cancer by 62% in women with BRCA 2 mutations.1 For BRCA carriers, yearly magnetic resonance imaging and mammograms beginning at the age of 25 years are recommended for breast cancer screening and have been shown to reduce mortality and cancer incidence. For ovarian cancer, the recommendation for surveillance is Ca-125 blood testing and pelvic ultrasonography every 6–12 months beginning at the age of 25–30 years in BRCA carriers.4
Many factors contribute to surgical decision making and management choices following BRCA testing. We and others have evaluated the behaviors of women with known deleterious mutations and the uptake of surveillance strategies, chemoprevention, and risk-reducing surgery in this population. There is far less known about the choices of women with a VUS. The objective of this study is to describe the behaviors of women who test positive for a VUS and directly compare their decision making and follow-up strategies with those of women with a known deleterious mutation. It is hypothesized that women with a VUS will have lower uptake of risk-reducing surgical interventions and that both groups of women in our study population will have lower utilization of ovarian cancer surveillance than breast cancer surveillance.
Materials and Methods
Kaiser Permanente Northern California (KPNC) is an integrated health organization with 58 rural and urban health facilities serving over 3 million members. Previous demographic studies have shown the KPNC population to be an excellent representative sample of the northern California population at large.10 Women who tested positive for a BRCA mutation from January 1995 to January 2012 were identified from a regional breast cancer tracking system for inclusion in the study. KPNC has specific standardized guidelines for appropriate referral to genetic counseling. Patients who meet these guidelines can be referred to genetic counseling. Initially this was done by paper consultation, but since 2008, it has been done electronically. These referral guidelines are posted on the consultation portal and are viewable by all KPNC practitioners in the electronic guideline library. The guidelines closely follow National Comprehensive Cancer Network guidelines for personal and family history of cancer and are updated every 2 years. The 2011 genetic referral guidelines are shown in Supplementary Table S1 online. Women who are identified with risk criteria including personal and family history were referred and seen by a genetic counselor, and risk assessment was performed with genetic testing offered based on risk assessment. Criteria for testing evolved over this time period and were individualized based on the available literature, size of family, and extent of history, but in general, ~10% risk of inherited cancer was used as a guide for testing. After testing positive for a BRCA mutation, women met with the genetic counselor and reviewed the significance of the result, and National Comprehensive Cancer Network guidelines, when available, for care were reviewed and posted in the chart. When national guidelines were not available, literature review was performed. Clinical management was performed by their local primary physician or obstetrician/gynecologist. All women were members of Kaiser Permanente Northern California for at least 1 year after testing and were older than 18 years. Women were excluded if they had ovarian cancer or a bilateral salpingo-oophorectomy prior to genetic testing. The study was approved by KPNC’s institutional review board.
A retrospective chart review was conducted to examine the health status and decision making of women who tested positive for either a deleterious BRCA mutation or a VUS. Primary outcomes were rates of RRM and RRSO among women with a deleterious mutation as compared with those among women with a VUS. Factors that were examined as contributing to decision making in variants included age at genetics testing, personal history of breast cancer, personal history of any cancer, menopausal status (defined as 1 year of amenorrhea), gravity and parity, any prior oral contraceptive usage, body mass index, family history of breast or ovarian cancer, type of variant, and estrogen receptor status of prior breast cancer. Secondary outcomes included compliance with breast and ovarian cancer surveillance and use of chemopreventive measures.
Compliance with surveillance guidelines for ovarian cancer was defined as at least one transvaginal ultrasound or Ca-125, assessed for each 12-month interval following the genetic test in women who had not undergone an RRSO. Compliance with screening guidelines for breast cancer was defined as at least one magnetic resonance imaging or mammogram in each 12-month interval following the genetic test in women who had not undergone bilateral mastectomy. This minimal measure of compliance was used in order to allow for the evolution in national recommendations and institutional guidelines over the 12-year time period. Patients who underwent RRSO or bilateral mastectomy or whose KPNC membership ended during a follow-up year were censored starting that year from the analyses of compliance with the appropriate surveillance strategy.
Demographic variables and clinical characteristics were examined in three sets of bivariate analysis. One bivariate analysis was performed to compare the women with a deleterious mutation with the women with a VUS. For the cohort of women with a VUS, two bivariate analyses were also performed comparing women who had RRSO versus those with no RRSO as well as those undergoing RRM versus those with no RRM, to examine demographic and clinical variables that could be predictive of surgical decision making. Categorical variables were evaluated using frequencies and proportions with χ2 tests and Fisher’s exact test for small cell sizes; continuously measured variables were evaluated using means and two-sided t-tests. Level of significance was set at a P value of 0.5. Medians with interquartile ranges were used to describe continuously non-normally distributed data. Odds ratios were calculated to compare RRSO, RRM, and surveillance methods in the women with a deleterious mutation versus women with a VUS. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Demographics
The demographic data for the two cohorts of women are presented in Table 1 . Women with a VUS were followed for a median of 69 months (range: 12–177 months), whereas the women with a deleterious mutation were followed for a median of 41 months (range: 22–66 months). There was no difference in mean age at testing between the 305 women with BRCA deleterious mutations (mean age: 47.3 years) and the 69 women with VUS (mean age: 50.7 years). The majority of women with a VUS were initially classified as “truly unknown” (79.7%), and only 3% of women with a VUS were classified as “suspect deleterious.” Women with deleterious mutations were more likely to be of Ashkenazi descent (19.7 vs. 5.8%; P = 0.0176). The majority of women in both groups were Caucasian, 62.3% for women with a VUS and 70.8% for women with deleterious mutations, which is not significantly different. The women with a VUS were more likely to have a prior diagnosis of breast cancer (76.8 vs. 58.4%; P = 0.0021) and to be menopausal (44.9 vs. 6.2%; P = 0.0001). There were similar rates of prior bilateral mastectomy (16.7% deleterious vs. 18.8% VUS), hysterectomy (1.3% deleterious vs. 5.8% VUS), and bilateral tubal ligation (7.5% deleterious vs. 5.8% VUS) between the two cohorts.
Risk reduction
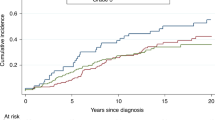
Rates of documented oral contraceptive usage were low for both groups of women, with utilization of 15.7 vs. 7.3% for women with a deleterious mutation and women with a VUS, respectively. Because the mean age of women in both groups was over 45 years, the use of oral contraceptives by these women in their childbearing years and duration of use weres not well documented. Thirty percent of women with a VUS underwent RRSO as compared with 74% of women with a deleterious mutation (odds ratio (OR): 6.4; P < 0.0001; Figure 1 ). The same trend was seen for RRM, with 44% of women with a deleterious mutation and 11% of women with a VUS undergoing this risk-reducing procedure. Of the demographic and clinical factors considered to impact decision making, none of the examined women were found to be associated with undergoing RRSO by bivariate analysis ( Table 2 ). The only factor found to be associated with undergoing RRM was the classification of variant type. Women with unknown mutations were less likely to have a RRM, whereas having a probable polymorphism or probably deleterious mutation was associated with RRM ( Table 3 ). Although family history of breast or ovarian cancer was not associated with RRSO, there was a trend toward a positive family history of breast cancer being associated with decision to undergo RRM.
Surveillance
Rates of breast and ovarian cancer surveillance were low for both cohorts immediately following BRCA testing as well as at 5 years after testing. In the first year after BRCA testing, women with a deleterious mutation were more likely to have mammograms (43 vs. 39%; OR: 2.12; 95% confidence interval (CI): 1.16–3.89), magnetic resonance imaging (35 vs. 15%; OR: 6.0; 95% CI: 2.69–13.37), Ca-125 testing (47 vs. 18%; OR: 7.67; 95% CI: 3.78–15.57), and transvaginal ultrasound (45 vs. 26%; OR: 4.95; 95% CI: 2.63–9.33) ( Figure 1 ). At 5 years after testing, rates of compliance with surveillance declined for both cohorts of women, with a greater drop off seen in the deleterious group ( Table 4 ).
Reclassification
During follow-up, 56% of VUS mutations were reclassified after a median of 39 months, longer than the median time to RRSO (18.6 months) or RRM (20.1 months) for this group of women. In the majority of women (84%) whose testing status was updated, the variant was classified to a more benign category (i.e., from “unknown” to “benign polymorphism” or from “suspect polymorphism” to “benign polymorphism”). Among the 30 women with “unknown” variants that were reclassified, 24 became benign polymorphisms. One unknown was upgraded to “suspect deleterious,” and another was reclassified as “deleterious.” Of the six women who underwent RRM, three were not reclassified, two were reclassified from unknown to benign polymorphism after surgery, and one was reclassified from probable polymorphism to benign polymorphism after surgery. Of the 21 women undergoing RRSO, 10 were never reclassified, 5 had unknown dates of reclassification, 4 had surgery after their VUS was reclassified to benign polymorphism, and 2 had surgery prior to being reclassified as benign polymorphism.
Discussion
In this community-based population study, we were able to demonstrate that women with a VUS had a greater than twofold lower likelihood of having risk-reducing surgery than women with a known deleterious mutation. Women with a VUS also had lower rates of surveillance than women with a deleterious mutation in the first few years after testing. Over time, the rates of surveillance steadily declined for women with a deleterious mutation, whereas the rates of surveillance for women with a VUS remained more stable, with higher rates of breast and ovarian cancer surveillance at 5 years after testing. It is unclear why women with a VUS would continue surveillance, although we could propose that the women with a deleterious mutation who are proactive about their health may choose risk-reducing surgery, and the smaller group who declined surgery may not be as compliant with surveillance practices. Whereas the recommendation for risk-reducing surgery in women with VUS is not clear, most women with VUS do not choose surgery, and therefore the group eligible for surveillance may include a greater proportion of women who want to be aggressive about prevention strategies.
We cannot evaluate how women with a VUS were counseled, if they were encouraged to do surveillance instead of surgery, or if some counselors reviewed the lack of evidence to support surveillance for ovarian cancer and actually discouraged ongoing surveillance with ultrasound and Ca-125. However, the genetic counselors used available literature and national guidelines to make clinical recommendations, and the fact that surveillance strategies were low in both the deleterious and the VUS groups makes it unlikely that genetic counselors would tell these women not to do surveillance. Surveillance strategies for ovarian cancer have not been shown to impact mortality,4 so it is possible that they were not encouraged or that patients chose not to do ovarian cancer surveillance because of the lack of data to support efficacy, but this does not explain why breast cancer surveillance was also poor in both groups.
Based on the factors that influenced referral, for example, a personal history of breast cancer, some women may have viewed their theoretical risk of cancer as still high despite the finding of a benign VUS and chosen surveillance or surgery based on their history. After counseling, others may have viewed their risk as low and chosen not to pursue risk-reducing strategies. Our study methods do not allow for interpretation of the reasons for surveillance and risk-reducing behavior.
The rates of risk-reducing surgery reported in this study are comparable to those in the two other reports that specifically examined the behaviors of women with a VUS.3,11 In contrast to those two studies from academic research programs, our study examines a population-based sample from a community health-care system in which patients are managed by their local physicians in consultation with genetics counselors and is more representative of what women are doing outside of research protocols and tertiary-care academic programs. In 2011, Murray et al.3 reported a 10.3% rate of RRM, similar to the rate of 11% in this study, and a rate of 20.6% for RRSO, which is lower than the rate of 30% in this study. In 2011, Ready et al.11 also found similar rates of RRM (11%), and lower rates of RRSO (10.6%) than in our study population. In their cohort, a personal history of breast cancer was associated with undergoing risk-reducing surgery and surveillance.11 We did not find the same association but did see a trend toward having a family history of breast cancer being associated with decision to undergo RRM.
Other reports examining risk-reducing behaviors among all women undergoing BRCA testing, including those who test positive for a VUS mutation, have reported conflicting data regarding impact of BRCA result on decision making. Mannis et al.12 reported testing positive for a deleterious BRCA mutation to have a high predictive value for women to undergo RRSO compared with women whose BRCA result was uninformative. Conversely, Loescher et al.13 found that the BRCA test result did not impact surveillance or RRSO rate among all women undergoing BRCA testing who were at risk for hereditary breast and ovarian cancers and found that provider’s recommendations played a large role in their cohort’s decision making. In our cohort, women with a deleterious mutation were much more likely to undergo RRSO than women with a VUS. However, among women with a VUS, we were not able to demonstrate any other clinical or demographic variables to be associated with undergoing RRSO.
Given the median age of 50 years in our VUS cohort compared with 45 years in the study by Murray et al.,3 women in our study were closer to menopause, which makes it more difficult to assess whether menopausal status or age could be a factor in decision making. It is also difficult to interpret the clinical significance of the factors we found associated with RRM, given the low number of women with a VUS undergoing this procedure (n = 6). However, all women with a VUS undergoing RRM had a family history of breast cancer. Reclassification rate was similarly high in the study by Murray et al.3 (44.9% over 9 years), but they were unable to assess timing of reclassification related to surgical decision making. In our analysis, the median time to reclassification was later than the median time that women with a VUS chose to undergo a risk-reducing surgery. Although several women had unknown mutations reclassified as benign following a surgical procedure, it is difficult to say whether knowing that negative result would have altered decision making, because many factors, such as family history, influence counseling and decision making.
Strengths of our study include the long median follow-up of 69 months for women with a VUS. Our data are also the first to be reported in the community setting, with test results and demographic and clinical characteristics based on the electronic medical record rather than patient recall. Our system also provides comprehensive care coverage, so that all testing and surveillance are accessible in a single electronic record. Limitations of our study include the length of time in which data were collected and the inability to capture individual counseling interviews and unique counselor or patient factors impacting decision making. Comparing the women with a deleterious mutation to the women with a VUS was one method to control for provider or patient variations in counseling. Further investigation into how women with a VUS are being counseled is warranted.
Although our study provides a description of the behavior of women with a VUS, the numbers of women undergoing RRSO and RRM are likely too small to detect factors that could be predictive of decision making. We also did not assess which factors patients feel are important in their decision making, which may be better assessed through patient survey.
In conclusion, our study shows that women with a VUS choose risk-reducing surgery less commonly than those with a deleterious mutation. However, management and decision making in this group remain complex, with many factors beyond BRCA result guiding care. In addition, VUS are often reclassified as benign but after the time that women are choosing to undergo risk-reducing surgery.
The current recommendations for counseling women with a VUS if there is no indication that the mutation is deleterious is to counsel on the basis of personal and family history risk assessment. Given that most of these women were reclassified to a more benign result, directing those women without strong family histories toward surveillance or bilateral salpingectomy as an alternative to oophorectomy at a young age as initial management strategies may balance risk reduction with the invasiveness of the intervention.
Disclosure
The authors declare no conflict of interest.
References
Hereditary breast and ovarian cancer syndrome ACOG Committee on Practice Bulletin. Obstet Gynecol 2009;113:6–11.
Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 2002;20:1480–1490.
Murray ML, Cerrato F, Bennett RL, Jarvik GP . Follow-up of carriers of BRCA1 and BRCA2 variants of unknown significance: variant reclassification and surgical decisions. Genet Med 2011;13:998–1005.
NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High Risk Assessment: Breast and Ovarian. Version 1.2010. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 13 December 2013.
Schwartz MD, Isaacs C, Graves KD, et al. Long-term outcomes of BRCA1/BRCA2 testing: risk reduction and surveillance. Cancer 2012;118:510–517.
Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, et al.; Hereditary Breast Cancer Clinical Study Group. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer 2008;122:2017–2022.
Beattie MS, Crawford B, Lin F, Vittinghoff E, Ziegler J . Uptake, time course, and predictors of risk-reducing surgeries in BRCA carriers. Genet Test Mol Biomarkers 2009;13:51–56.
Friebel TM, Domchek SM, Neuhausen SL, et al. Bilateral prophylactic oophorectomy and bilateral prophylactic mastectomy in a prospective cohort of unaffected BRCA1 and BRCA2 mutation carriers. Clin Breast Cancer 2007;7:875–882.
Metcalfe KA, Lubinski J, Ghadirian P, et al.; Hereditary Breast Cancer Clinical Study Group. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: the Hereditary Breast Cancer Clinical Study Group. J Clin Oncol 2008;26:1093–1097.
Gordon NP . A Comparison of Sociodemographic and Health Characteristics of the Kaiser Permanente Northern California Membership Derived from Two Data Sources: The 2008 Member Health Survey and the 2007 California Health Interview Survey. Internal Division of Research report. Oakland, CA: Kaiser Permanente Division of Research; 2012 Jan. http://www.dor.kaiser.org/external/chis_mhs_comparison_2008/ Accessed 4 January 2014.
Ready K, Gutierrez-Barrera AM, Amos C, et al. Cancer risk management decisions of women with BRCA1 or BRCA2 variants of uncertain significance. Breast J 2011;17:210–212.
Mannis GN, Fehniger JE, Creasman JS, Jacoby VL, Beattie MS . Risk-reducing salpingo-oophorectomy and ovarian cancer screening in 1077 women after BRCA testing. JAMA Intern Med 2013;173:96–103.
Loescher LJ, Lim KH, Leitner O, Ray J, D’Souza J, Armstrong CM . Cancer surveillance behaviors in women presenting for clinical BRCA genetic susceptibility testing. Oncol Nurs Forum 2009;36:E57–E67.
Acknowledgements
Funding for this project was provided by a Kaiser Permanente 2012 community benefit grant.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Table S1
(DOC 229 kb)
Rights and permissions
About this article
Cite this article
Garcia, C., Lyon, L., Littell, R. et al. Comparison of risk management strategies between women testing positive for a BRCA variant of unknown significance and women with known BRCA deleterious mutations. Genet Med 16, 896–902 (2014). https://doi.org/10.1038/gim.2014.48
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2014.48
Keywords
This article is cited by
-
Experiences of individuals with a variant of uncertain significance on genetic testing for hereditary cancer risks: a mixed method systematic review
Journal of Community Genetics (2022)
-
Analysis of BRCA1/2 mutation spectrum and prevalence in unselected Chinese breast cancer patients by next-generation sequencing
Journal of Cancer Research and Clinical Oncology (2017)
-
Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients
Scientific Reports (2016)
-
Investigating the effect of 28 BRCA1 and BRCA2 mutations on their related transcribed mRNA
Breast Cancer Research and Treatment (2016)