Abstract
To fulfill the purpose of newborn screening, comprehensive newborn screening programs must ensure that infants and children with newborn screening conditions are not only diagnosed but also they maintain engagement in appropriate lifespan and family-centered care for best outcomes. To ensure success, monitoring and care-coordination requires a systems-based approach to streamline the significant surveillance activities, which must not overburden the critical core functions of newborn screening nor the health care delivery system. Furthermore, treatment and care can only be improved by translating reliable knowledge into changes in practice, a process that requires evaluations of outcomes that are confirmable at the local level and translatable into a larger, e.g., national data set. We describe a sustainable public health systems approach to long-term follow-up, built on existing comprehensive newborn screening infrastructure and compatible with national endeavors. We also describe early experience with implementation of a centralized public-health tracking model and show that a significant proportion of cases detected through newborn screening do not continue with subspecialty care as they get older.
Similar content being viewed by others
OVERVIEW
The purpose of the public health service of newborn screening (NBS) is to identify infants who will benefit from early intervention to reduce death, mental retardation, and other significant health problems and to engage these babies with effective care. NBS only reaches its full potential when infants with positive screens have access to diagnostic evaluations and when infants with confirmed diagnoses have access to appropriate lifespan family-centered care.
It is widely accepted that public-health-based NBS is a model of success for universal access to US health care, with >99%1 of all live born infants being screened and 49 of 50 states reporting that infants are followed up or “tracked” for diagnosis and treatment.2 It is also widely accepted that the NBS program should be extended to encompass follow-up activities beyond the initial diagnosis and treatment.3 However, “such activities have long been an underfunded and comparatively neglected component of the NBS system.”4 Families move, change health care providers, become lost to follow–up, and long-term outcomes are not documented for many. This opens the door to late development of poor outcomes, including death, and leaves an incomplete loop in program quality assurance.
Comprehensive state-based NBS programs provide a centralized system for ensuring that all infants are screened, for identifying affected infants, and for tracking affected infants to treatment.5 To fulfill the public health purpose, a comprehensive NBS program must include long-term follow-up (LTFU) activities that monitor for universal continuity of quality care. Centralized NBS LTFU program activities offer the opportunity to include feedback on outcomes to clinical centers to enhance quality improvements. Other activities of a centralized NBS LTFU program may also include database management for the purpose of related studies, provided this is done with appropriate human subjects review. Such data management might include notification of opportunities for enrolling in clinical trials and dissemination of information on new or emerging treatments; al though public health service is not typically focused on promoting research, at least one advantage of such regulated information sharing is that educational information would be distributed universally by a central agency. The infrastructure and authority for coordinating these services is largely in place in all of the comprehensive state-based NBS programs.
Use of an established NBS data set such as that at the New England NBS Program (NENSP) allows linkage to many core NBS laboratory and clinical data for five of the six New England states and provides a model for a technical framework for states to enhance their local follow-up systems. We describe our preliminary experience with implementation of LTFU activities for individuals who are followed up for cystic fibrosis (CF), hemoglobinopathies, and metabolic conditions.
Public health model
The model was developed using Massachusetts' centralized state-based comprehensive NBS program. The extension of the state model is based on the formal relationships that exist between state departments of public health in existing regional NBS programs such as the NENSP.
The model builds on the capacity of our New England states' universal NBS and follow-up programs to ensure that (a) all children diagnosed with NBS conditions have access to and continue with appropriate care and treatment for their condition and (b) to ensure that the definition of “appropriate care and treatment” is evidence based, using sound epidemiological methods to improve our understanding of natural and treatment histories associated with these conditions, thus our ability to improve care-and-treatment outcomes relative to the natural history of the condition.
Essential components of the model
-
1
Centralized NBS program, inclusive of laboratory, follow–up, and data system.
-
2
Authority to perform state-based NBS activities, inclusive of follow-up.
-
3
A relational database for linkage between data from the activities of NBS laboratory, reporting, short-term follow-up and LTFU, complete with standardized drop-down menus and standards for data entry.
-
4
Established formal lines of communication between the central NBS program, the multiple clinical centers, and the medical homes of cases. In addition to lines of communication for basic NBS functions, the formation of condition-specific workgroups facilitates (a) developing of variables outlined in points 5 and 6 and (b) reporting of aggregate data for quality improvements.
-
5
Specific case definitions and characteristics of patients followed up will be included in LTFU activities.
-
6
Specific variables to be collected in minimum and maximum data sets.
-
7
For extended model: authority and policies for integrating multistate data.
Mode of enrollment
On notification by a qualified diagnostic center that an infant meets the case definition for the NBS condition, NBS program staff revise the infant status in the central database from “screen positive” to “case” and enter the infant into the LTFU module that is linked to the NBS database. The LTFU module includes laboratory and clinical data supporting the case determination (Fig. 1) and the name of the specific qualified diagnostic center.
Public health authority for inclusion and data collection
The model makes use of public health authority for data collection by a centralized NBS program (e.g., a state-based NBS program) for quality assurance of the public health service. Although consent-based models exist, projected advantages of the public health model include assurance of appropriate denominator and lower likelihood of outcome bias.
Evidence-based quality improvements
Aggregate data are presented to condition-specific workgroups for review, comment, and possible further investigation. Particularly, relevant aggregate data are summarized and communicated to medical homes of relevant cases by fax and phone.
METHODS AND IMPLEMENTATION
Exercise of public health authority for data collection
Massachusetts recognized a new level of need for evidence-based public health decision making and quality assurance of the NBS program in its 1999 expansion of the NBS panel.6 In addition to activities within the two statewide studies, efforts to ensure similar quality assurance activities for infants and children diagnosed with conditions that were not included in either study were begun. Efforts were dependent on voluntary submission of data by subspecialty clinical practice centers. As of December 12, 2008, changes to Massachusetts NBS Regulations require that health care providers respond to requests for LTFU information by the NBS program for the purposes of quality assurance, quality improvement, and ongoing evaluation of the effectiveness of NBS (105 CMR 270.010). In Maine, interpretation of state rules allowed health care providers to voluntarily report long-term and short-term outcome data to the Maine NBS program.
Minimum data set data elements and data collection
The NENSP tracked a series of “census” data elements common to LTFU for all conditions; these included the following: aliases and current names of mother and child, initial and current maternal address and phone, initial and current primary care provider, initial and current specialty care provider, and date of last specialty clinic visit. Data collection began by surveys faxed to each specialty with a list of children who were last known to be followed up at that center with request for confirmation of continued care and date of last clinic visit. Some specialty centers also provided lists of current patients and date of last visit. For all children thought to be alive who were not known to any one of the specialty centers, surveys were faxed and telephoned to the child's last known primary care provider requesting information about continuation of specialty care.
Subjects included in LTFU
All individuals born since February 1, 1999, and diagnosed with CF, a sickling hemoglobinopathy, or a metabolic condition who were identified as a result of NBS testing were included in LTFU in Massachusetts. All individuals born in Maine since September 1, 1999, and diagnosed with medium chain acyl-CoA dehydrogenase deficiency (MCADD) or born in Maine since July 1, 2001, and diagnosed with one of the other metabolic conditions detected through the NBS program were included.
RESULTS
Case distribution
Table 1 shows the distribution of identified cases by year of birth and specific to condition. Approximately 40% of all cases had metabolic conditions, 23% had CF, and 37% had a sickling hemoglobinopathy.
Indications from current census data
Two percent of all cases identified by NBS are no longer alive (Table 2). All deaths of metabolic cases were directly attributable to their condition, and deaths of CF cases seem to have been related to CF (e.g., bowel obstruction). At least two of three deaths of cases with sickling hemoglobinopathies seem to be unrelated to the sickling condition.
Of the remaining 933 cases, there were no current LTFU data available for 136 (15%); 53 of these cases were documented to have moved out of state or out of country, and 83 (with current ages ranging from 3 to 10 years) were lost to follow-up at various points of time postdiagnosis despite continued efforts. Confirmed diagnoses or initial treatments identified the site of specialty care, where follow-up efforts focused; such efforts eventually yielded success for 576 cases. For another 221, the only reliable source of LTFU information was from the primary care provider; the largest proportion of cases where the primary care provider was the only source of current information was seen among metabolic cases (51%). Numerous cases transferred their care from one subspecialty center to another; 50% of metabolic cases and 28% of CF cases transferred care at least once, whereas only 5% of cases with sickling hemoglobinopathies transferred care.
Table 2 also shows summary information relating to utilization of subspecialty care. Of interest is that as many as 36% of metabolic cases had not been seen by a subspecialist in more than 2 years, whereas 90% or more of CF and sickling hemoglobinopathy cases continue with subspecialty care.
Annual trends in utilization of subspecialty care
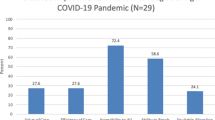
Figure 2 shows that aged as young as 3 years, a significant proportion of children diagnosed with fatty acid oxidation disorders, organic acidemias, and amino acid disorders were not clinically evaluated by a subspecialist, even on an annual basis. For children with urea cycle disorders or with classical galactosemia, the decrease in subspecialty care seems to be delayed until school age. In general, more than 90% of children diagnosed with CF and more than 80% of children diagnosed with a sickling hemoglobinopathy continue with subspecialty care for as long as they have been followed up (up to 10 years). The specific metabolic conditions diagnosed among children who had not been evaluated by a subspecialist in more than 2 years are varied and included conditions believed to be associated with poor outcomes (Table 3).
Demonstrated opportunities for quality improvements
Two examples of a quality improvement use of aggregate data from a complete and unbiased centralized data set are notable. Centralized LTFU of metabolic cases uncovered a severe hypoglycemic episode of one and the late-occurring deaths of another two children with MCADD.7 In addition and before the peer reviewed publication, the NENSP notified primary care providers of MCADD cases by phone or fax of the documented risk to MCADD patients, despite NBS. Similarly, centralized LTFU of CF cases or borderline diagnoses uncovered pulmonary signs and symptoms in children carrying a genotype that had been thought to be benign8 and in children with inconclusive sweat test results. These data were shared with health care providers responsible for infants and children with this genotype (Fig. 3). Directly as a result of each notification, additional visits with subspecialists were scheduled, and one infant who had not been seeing a subspecialist after a borderline diagnosis received a confirmed CF diagnosis and is now treated.
DISCUSSION
We describe the foundation of a sustainable systematic public health approach to LTFU designed to ensure (1) a high level of quality assurance for lifespan care, (2) access to cleaned and unbiased outcome data for infants and children with NBS conditions for which natural histories or treatment outcomes are not well understood, and (3) evidence-based quality improvements. The system derives benefit from public health authority to collect centralized data, which allows a central authority to monitor whether a patient who is no longer followed up at one center obtains care at another center, to ensure appropriate denominators (avoidance of replicate counting as a by-product of center transfer and ensuring inclusivity to avoid denominator bias that might otherwise remain uncovered), and to use established lines of communication to distribute information for quality improvements.
We also describe some of our preliminary experience with this approach, as applied to sickling hemoglobinopathies, CF, and metabolic disorders after 9 years of voluntary data reporting and only 1 year requiring reporting in Massachusetts. These three sets of conditions provide models with either sufficient prevalence in heterogeneous populations for comparative analyses or relatively insufficient data on the clinical utility of screening.
A fair number of cases (9%) have been lost to LTFU (lost) despite significant efforts to find these children. These lost cases are distinct from those known to have moved out of the New England region or out of country (many of those are known to have transferred to an out-of-state specialty center). We only know that they are not seen in a subspecialty clinic. The lost cases may remain in Massachusetts or Maine with no new primary care provider or having changed primary care provider.
Subspecialists do not see a significant and increasing proportion of cases diagnosed with metabolic conditions as the cases get older. In contrast, subspecialists continue to see the majority of cases diagnosed with CF or a sickling hemoglobinopathy (Fig. 2). Conventional expectations for quality care of any rare condition would include routine evaluations by a subspecialist, particularly for rare metabolic conditions thought to present with little warning. Informal reports from 23 primary care providers about parent reasoning for discontinuing subspecialty care include complaints of it being too far to travel (n = 4), seeing no advantage to subspecialty follow-up because child receives no specific treatment (n = 18), belief that the child was doing well (n = 7), recognition that when a condition was biochemically defined, the subspecialist could not clarify a spectrum of disease or confirm that treatment would be beneficial (n = 6), or feeling that evaluation performed at a specialty clinic was unnecessarily intrusive and caused anxiety (n = 5). Because the geographic distribution of the four metabolism and genetics clinics is similar to that of the CF and hemoglobin clinics, it is unlikely that travel alone is a major factor in the discrepancy for utilization of specialty care. Although the infrastructure afforded in CF clinics by the CF foundation is strong, the absence of such an infrastructure among the hemoglobin clinics, which also show a high rate of continued subspecialty care, suggests that this is not the major factor in the discrepancy. It seems likely that the continuity of subspecialty care for children with CF and hemoglobinopathies may be because of (1) a compromised clinical status of the child and/or (2) a general awareness of the condition and acceptance that ongoing adherence to proven treatments are required to obtain a good quality of life.
An important consideration in the development of any LTFU model is that the only reliable source of information about when and where subspecialty care occurred was the primary care provider (in 28% of all cases and in 51% of metabolic cases in particular). One can infer that LTFU models relying mainly on reports from subspecialty centers may suffer from incomplete data. Outcome data are likely to be compromised by a confounding bias in the absence of a public health model that ensures complete denominators and lower likelihood of bias in numerators. Furthermore, and particularly for metabolic conditions, if the observed discontinuation of subspecialty care visits remains true, then the knowledge base about these conditions will not improve unless researchers, providers, and families can ensure a reasonable mechanism to obtain reliable long-term outcome data. Condition-specific workgroups should work to establish clinical recommendations for minimum requirements for timing of well visits to subspecialty care, to define well-care standard protocols, to establish minimum requirements for sick-care protocols, and to define data-reporting requirements from sick-care visits.
Despite the necessary foundation of the centralized database and infrastructure already in place in NBS programs, such LTFU efforts are labor intensive, burdened by center-to-center transfers, and further complicated by undocumented changes in primary health care providers, and the caseload is cumulative. In the future, LTFU efforts might benefit from automated health information exchange (HIE) that interacts with the centralized population-based data set. In addition, until such an HIE is in place to facilitate tracking to a current medical home, a practice change to manually identify (to the state NBS program/central data set) the new health care provider of an infant/child with a NBS condition is warranted. Because sick visits are less predictable and may occur outside of the medical home, HIE that includes, e.g., emergency room access would ensure quality care and complete data for long-term monitoring.
Finally, although national efforts to aggregate LTFU data on particularly rare conditions should be applauded, the value of state-based efforts must not be underestimated. State stewardship of confidential data has in general been exemplary. State privacy laws may be more stringent than Health Insurance Portability and Accountability Act, inhibiting interstate data collection and sharing. State authority for public health surveillance may permit accurate denominator and numerator data on state-based data that is unavailable from national data sets. State NBS programs have established relationships with primary care providers and treatment centers, are familiar with social and professional mores in their local region, and have access to multiple data sets and tools to assist in the location and assistance of cases. Recognition of trends is often most readily apparent when analysis is applied to data collected under highly standardized conditions, such as that attainable under state public health authority. Hypotheses generated by trends observed at the state level can then be expanded into quality national research efforts to improve the well being of individuals with NBS conditions.
REFERENCES
National Newborn Screening and Genetics Resource Center, 2010. Available at: http://genes-r-us.uthscsa.edu/. Accessed July 30, 2009.
Components included in newborn screening follow-up in the US in 2010. Available at: http://www2.uthscsa.edu/nnsis/. Accessed July 30, 2009.
Newborn Screening Task Force Convened in Washington, DC, May 10–11, 1999. Serving the family from birth to the medical home. Pediatrics 2000: 106: 383–427.
Association of Maternal and Child Health Programs. Newborn screening long-term follow-up assessment, 2007. Available at: http://www.amchp.org/publications/ChildrensHealth/Documents/NBS-LTFU%20Assessment.pdf. Accessed July 30, 2009.
Tuerck J, Dhondt JL, King P, et al. Newborn screening follow up: approved guideline. Clinical and Laboratory Standards Institute document I/LA27-A. Wayne, PA: Clinical and Laboratory Standards Institute, 2006.
Atkinson K, Zuckerman B, Sharfstein JM, Levin D, Blatt RJR, Koh HK . A public health response to emerging technology: expansion of the Massachusetts Newborn Screening Program. Public Health Rep 2001; 116: 122–131.
Hsu H-W, Zytkovicz TH, Comeau AM, et al. Spectrum of medium chain acyl-CoA dehydrogenase (MCAD) deficiency detected by newborn screening. Pediatrics 2008; 121: e1108–e1114.
O'Sullivan BP, Zwerdling R, Dorkin HL, Comeau AM, Parad RB . Early pulmonary manifestation of cystic fibrosis in children with the ΔF508/R117H–7T genotype. Pediatrics 2006; 118: 1260–1265.
Acknowledgements
This work was funded in part by HRSA Grants H46MC00198 and U22MC03959 subcontract for Priority Focus 2. The authors thank Mss. Claire Hughes, Joyce Bailey, and Jane Griffin for their data collection and entry work, the Clinical Directors of the Massachusetts CF, HgB, and Metabolism clinics and the Maine Genetics clinics for facilitating reports to the respective newborn screening programs, and the NBS Regional Long-Term Follow-up Workgroup for their continued work in developing a centralized LTFU system.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sahai, I., Eaton, R., Hale, J. et al. Long-term follow-up to ensure quality care of individuals diagnosed with newborn screening conditions: Early experience in New England. Genet Med 12 (Suppl 12), S220–S227 (2010). https://doi.org/10.1097/GIM.0b013e3181fe5d37
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e3181fe5d37
Keywords
This article is cited by
-
Care Coordination: Empowering Families, a Promising Practice to Facilitate Medical Home Use Among Children and Youth with Special Health Care Needs
Maternal and Child Health Journal (2018)