Abstract
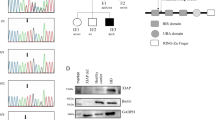
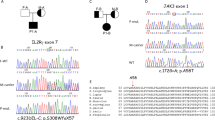
Lymphocyte apoptosis is mainly induced by either death receptor-dependent activation of caspase-8 or mitochondria-dependent activation of caspase-9. Mutations in caspase-8 lead to autoimmunity/lymphoproliferation and immunodeficiency. This work describes a heterozygous H237P mutation in caspase-9 that can lead to similar disorders. H237P mutation was detected in two patients: Pt1 with autoimmunity/lymphoproliferation, severe hypogammaglobulinemia and Pt2 with mild hypogammaglobulinemia and Burkitt lymphoma. Their lymphocytes displayed defective caspase-9 activity and decreased apoptotic and activation responses. Transfection experiments showed that mutant caspase-9 display defective enzyme and proapoptotic activities and a dominant-negative effect on wild-type caspase-9. Ex vivo analysis of the patients’ lymphocytes and in vitro transfection experiments showed that the expression of mutant caspase-9 correlated with a downregulation of BAFFR (B-cell-activating factor belonging to the TNF family (BAFF) receptor) in B cells and ICOS (inducible T-cell costimulator) in T cells. Both patients carried a second inherited heterozygous mutation missing in the relatives carrying H237P: Pt1 in the transmembrane activator and CAML interactor (TACI) gene (S144X) and Pt2 in the perforin (PRF1) gene (N252S). Both mutations have been previously associated with immunodeficiencies in homozygosis or compound heterozygosis. Taken together, these data suggest that caspase-9 mutations may predispose to immunodeficiency by cooperating with other genetic factors, possibly by downregulating the expression of BAFFR and ICOS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Nagata S . Apoptosis by death factor. Cell 1997; 88: 355–365.
Cohen GM . Caspases: the executioners of apoptosis. J Biochem 1997; 326: 1–16.
Dianzani U, Chiocchetti A, Ramenghi UX . Role of inherited defects decreasing Fas function in autoimmunity. Life Sci 2003; 72: 2803–2824.
Zou H, Li Y, Liu X, Wang X . An APAF-1-cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 1999; 274: 11549–11556.
Riedl SJ, Shi Y . Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 2004; 5: 897–907.
Li H, Zhu H, Xu CJ, Yuan J . Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94: 491–501.
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X . Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481–490.
Budd RC . Death receptors couple to both cell proliferation and apoptosis. J Clin Invest 2002; 109: 437–441.
Alam A, Cohen LY, Aouad S, Sekaly RP . Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med 1999; 190: 1879–1890.
Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY et al. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci USA 2008; 105: 3515–3520.
Sordet O, Rébé C, Plenchette S, Zermati Y, Hermine O, Vainchenker W et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood 2002; 100: 4446–4453.
Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol 2004; 173: 2976–2984.
Yi CH, Yuan J . The Jekyll and Hyde functions of caspases. Dev Cell 2009; 16: 21–34.
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 2002; 419: 395–399.
Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev 2003; 17: 883–895.
Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K et al. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell 1998; 1: 553–563.
Dohrman A, Kataoka T, Cuenin S, Russell JQ, Tschopp J, Budd RC . Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-kappa B activation. J Immunol 2005; 174: 5270–5278.
Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 2005; 307: 1465–1468.
Woo M, Hakem R, Furlonger C, Hakem A, Duncan GS, Sasaki T et al. Caspase-3 regulates cell cycle in B cells: a consequence of substrate specificity. Nat Immunol 2003; 4: 1016–1022.
Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M et al. Inherited human caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell 1999; 98: 47–58.
Fisher GN, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY et al. Dominant interfering Fas gene mutations impair apoptosis in a human lymphoproliferative syndrome. Cell 1995; 81: 935–946.
Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 1995; 268: 1347–1349.
Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD . Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest 1996; 98: 1107–1113.
Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood 2010; 116: e35–e40.
Dianzani U, Bragardo M, Di Franco D, Alliaudi C, Scagni P, Buonfiglio D et al. Deficiency of the Fas apoptosis pathway without Fas gene mutations in pediatric patients with autoimmunity/lymphoproliferation. Blood 1997; 89: 2871–2879.
Ramenghi U, Bonissoni S, Migliaretti G, DeFranco S, Bottarel F, Gambaruto C et al. Deficiency of the Fas apoptosis pathway without Fas gene mutations is a familial trait predisposing to development of autoimmune diseases and cancer. Blood 2000; 95: 3176–3182.
Campagnoli MF, Gambarini L, Quarello P, Garelli E, Carando A, Baravalle V et al. The broad spectrum of autoimmune lymphoproliferative disease: molecular bases, clinical features and long-term follow-up in 31 patients. Haematologica 2006; 91: 538–541.
Cunningham-Rundles C, Bodian C . Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol 1999; 92: 34–48.
Park MA, Li JT, Hagan JB, Maddox DE, Abraham RS . Common variable immunodeficiency: a new look at an old disease. Lancet 2008; 372: 489–502.
Warnatz K, Schlesier M . Flow cytometric phenotyping of common variable immunodeficiency. Cytometry B Clin Cytom 2008; 74: 261–271.
Rensing-Ehl A, Warnatz K, Fuchs S, Schlesier M, Salzer U, Draeger R et al. Clinical and immunological overlap between autoimmune lymphoproliferative syndrome and common variable immunodeficiency. Clin Immunol 2010; 137: 357–365.
Park JH, Resnick ES, Cunningham-Rundles C . Perspectives on common variable immune deficiency. Ann NY Acad Sci 2011; 1246: 41–49.
Gholam C, Grigoriadou S, Gilmour KC, Gaspar HB . Familial haemophagocytic lymphohistiocytosis: advances in the genetic basis, diagnosis and management. Clin Exp Immunol 2011; 163: 271–283.
Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA . Perforin: structure, function, and role in human immunopathology. Immunol Rev 2010; 235: 35–54.
Rezaei N, Mahmoudi E, Aghamohammadi A, Das R, Nichols KE . X-linked lymphoproliferative syndrome: a genetic condition typified by the triad of infection, immunodeficiency and lymphoma. Br J Haematol 2011; 152: 13–30.
Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet 2005; 37: 820–828.
Clementi R, Chiocchetti A, Cappellano G, Cerutti E, Ferretti M, Orilieri E et al. Variations of the perforin gene in patients with autoimmunity/lymphoproliferation and defective Fas function. Blood 2006; 108: 3079–3084.
Clementi R, Locatelli F, Dupre L, Garaventa A, Emmi L, Bregni M et al. A proportion of patients with lymphoma may harbor mutations of the perforin gene. Blood 2005; 105: 4424–4428.
Orilieri E, Cappellano G, Clementi R, Cometa A, Ferretti M, Cerutti E et al. Variations of the perforin gene in patients with type 1 diabetes. Diabetes 2008; 57: 1078–1083.
Renatus M, Stennicke HR, Scott FL, Liddington RC, Salvesen GS . Dimer formation drives the activation of the cell death protease caspase 9. Proc Natl Acad Sci USA 2001; 98: 14250–14255.
Adams JM, Cory S . Apoptosomes: engines for caspase activation. Curr Opin Cell Biol 2002; 14: 715–720.
Pop C, Timmer J, Sperandio S, Salvesen GS . The apoptosome activates caspase-9 by dimerization. Mol Cell 2006; 22: 269–275.
Ehrenfeld M, Abu-Shakra M, Buskila D, Shoenfeld Y . The dual association between lymphoma and autoimmunity. Blood Cells Mol Dis 2001; 27: 750–756.
Oertel SH, Riess H . Immunosurveillance, immunodeficiency and lymphoproliferations. Recent Results Cancer Res 2002; 159: 1–8.
Muris JJ, Cillessen SA, Vos W, van Houdt IS, Kummer JA, van Krieken JH et al. Immunohistochemical profiling of caspase signaling pathways predicts clinical response to chemotherapy in primary nodal diffuse large B-cell lymphomas. Blood 2005; 105: 2916–2923.
Shulga-Morskaya S, Dobles M, Walsh ME, Ng LG, MacKay F, Rao SP et al. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J Immunol 2004; 173: 2331–2341.
Xu H, Li X, Liu D, Li J, Zhang X, Chen X et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 2013; 496: 523–527.
Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 2000; 404: 995–999.
Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS . Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity 2003; 18: 279–288.
Boggio E, Aricò M, Melensi M, Dianzani I, Ramenghi U, Dianzani U et al. Mutation of FAS, XIAP, and UNC13D genes in a patient with a complex lymphoproliferative phenotype. Pediatrics 2013; 132: e1052–e1058.
Aricò M, Boggio E, Cetica V, Melensi M, Orilieri E, Clemente N et al. Variations of the UNC13D gene in patients with autoimmune lymphoproliferative syndrome. PLoS One 2013; 8: e68045.
Voskoboinik I, Thia MC, Trapani JA . A functional analysis of the putative polymorphisms A91V and N252S and 22 missense perforin mutations associated with familial hemophagocytic lymphohistiocytosis. Blood 2005; 105: 4700–4706.
Zheng TS, Hunot S, Kuida K, Flavell RA . Caspase knockouts: matters of life and death. Cell Death Differ 1999; 6: 1043–1053.
Acknowledgements
This work was partially supported by Associazione Italiana Ricerca sul Cancro (AIRC, Milan; funding number #14430), Fondazione Cariplo (Milan), Fondazione Italiana Sclerosi Multipla (FISM, Rome), Compagnia di San Paolo (Turin), Fondazione Cassa di Risparmio di Cuneo (Cuneo), Regione Piemonte (Turin) and Associazione ‘Amici di Jean’ (Turin). We thank Dr Michael Lenardo and Dr Frederic Rieux-Laucat for providing some patients’ DNA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Genes and Immunity website
Supplementary information
Rights and permissions
About this article
Cite this article
Clemente, N., Boggio, E., Gigliotti, C. et al. A mutation in caspase-9 decreases the expression of BAFFR and ICOS in patients with immunodeficiency and lymphoproliferation. Genes Immun 16, 151–161 (2015). https://doi.org/10.1038/gene.2014.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2014.74
This article is cited by
-
In search of genetic factors predisposing to familial hairy cell leukemia (HCL): exome-sequencing of four multiplex HCL pedigrees
Leukemia (2020)
-
Key apoptotic genes APAF1 and CASP9 implicated in recurrent folate-resistant neural tube defects
European Journal of Human Genetics (2018)