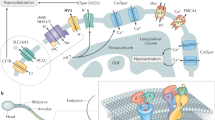
Abstract
Look at songs, hidden in eggs. Carl Sandburg in Prairie
Fertilization is the process by which sperm and egg unite. An expanded understanding of the mechanisms that underlie these events has provided insights into an important aspect of early development and also has proven to be a valuable model in which to study cellular function. In addition, many emerging strategies for contraception and for the treatment of infertility are based on the mechanism of gamete interaction. Here, we discuss the cell and molecular biology of mammalian fertilization, highlight selected recent breakthroughs and attempt to identify key unanswered questions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wilson, E.B. The cell in development and heredity 1–1232 (Macmillan, New York 1925).
Maller, J.L. Maturation promoting factor in the early days. Trends Biochem. Sci. 20, 524–528 (1995).
Drewett, J.G. & Garbers, D.L. The family of guanylyl cyclase receptors and their ligands. Endocr. Rev. 15, 135–162 (1994).
Chen, Y. et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628 (2000).
Primakoff, P. & Myles, D.G. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 16, 83–87 (2000).
U.S. Census Bureau World Popclock. http://www.census.gov/cgi-bin/ipc/popclockw (2002).
Wassarman, P.M., Jovine, L. & Litscher, E.S. A profile of fertilization in mammals. Nature Cell Biol. 3, E59–E64 (2001).
Wassarman, P.M. & Florman, H.M. Cellular mechanisms during mammalian fertilization. Handbook of Physiology: Section 14- Cell Physiology 885–938 (Oxford University Press, New York, 1997).
Yanagimachi, R. The Physiology of Reproduction 189–317 (Raven Press, New York 1994).
Myles, D.G. & Primakoff, P. Why did the sperm cross the cumulus – to get to the oocyte: functions of the sperm surface proteins PH-20 and fertilin in arriving at and fusing with the egg. Biol.Reprod. 56, 320–327 (1997).
Snell, W.J. & White, J.M. The molecules of mammalian fertilization. Cell 85, 629–637 (1996).
Vacquier, V.D. Evolution of gamete recognition proteins. Science 281, 1995–1998 (1998).
Visconti, P.E. et al. The molecular basis of sperm capacitation. J. Androl. 19, 242–248 (1998).
Florman, H.M., Arnoult, C., Kazam, I.G., Li, C. & O'Toole, C.M.B. An intimate biochemistry: egg-regulated acrosome reactions of mammalian sperm. Adv. Devel. Biochem. 5, 147–186 (1999).
Visconti, P.E. et al. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 53, 133–150 (2002).
Wiesner, B. et al. Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. J. Cell Biol. 142, 473–484 (1998).
Ho, H.C. & Suarez, S.S. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction 122, 519–526 (2001).
Arnoult, C. et al. Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc.Natl Acad. Sci. USA 96, 6757–6762 (1999).
Visconti, P.E. et al. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev. Biol. 214, 429–443 (1999).
Contreras, H.R. & Llanos, M.N. Detection of progesterone receptors in human spermatozoa and their correlation with morphological and functional properties. Int. J. Androl. 24, 246–252 (2001).
Bleil, J.D. & Wassarman, P.M. Autoradiographic visualization of the mouse egg's sperm receptor bound to sperm. J. Cell Biol. 102, 1363–1371 (1986).
Bleil, J.D. & Wassarman, P.M. Mammalian sperm–egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 20, 873–882 (1980).
Florman, H.M. & Wassarman, P.M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell 41, 313–324 (1985).
Wassarman, P.M. & Litscher, E.S. Towards the molecular basis of sperm and egg interaction during mammalian fertilization. Cells Tissues Organs 168, 36–45 (2001).
Easton, R.L. et al. Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sda antigen. J. Biol. Chem. 275, 7731–7742 (2000).
Hardy, D.M. & Garbers, D.L. A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J. Biol. Chem. 270, 26025–26028 (1995).
Hardy, D.M. & Garbers, D.L. Species-specific binding of sperm proteins to the extracellular matrix (zona pellucida) of the egg. J. Biol. Chem. 269, 19000–19004 (1994).
Chen, J., Litscher, E.S. & Wassarman, P.M. Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc. Natl Acad. Sci. USA 95, 6193–6197 (1998).
Macek, M.B., Lopez, L.C. & Shur, B.D. Aggregation of β-1,4-galactosyltransferase on mouse sperm induces the acrosome reaction. Dev. Biol. 147, 440–444 (1991).
Gong, X., Dubois, D.H., Miller, D.J. & Shur, B.D. Activation of a G protein complex by aggregation of β-1,4-galactosyltransferase on the surface of sperm. Science 269, 1718–1721 (1995).
Koyota, S., Wimalasiri, K.M. & Hoshi, M. Structure of the main saccharide chain in the acrosome reaction-inducing substance of the starfish, Asterias amurensis. J. Biol. Chem. 272, 10372–10376 (1997).
Hirohashi, N. & Vacquier, V.D. High molecular mass egg fucose sulfate polymer is required for opening both Ca2+ channels involved in triggering the sea urchin sperm acrosome reaction. J. Biol. Chem. 277, 1182–1189 (2002).
Arnoult, C., Cardullo, R.A., Lemos, J.R. & Florman, H.M. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc. Natl Acad. Sci. USA 93, 13004–13009 (1996).
Ward, C.R., Storey, B.T. & Kopf, G.S. Selective activation of Gi1 and Gi2 in mouse sperm by the zona pellucida, the egg's extracellular matrix. J. Biol. Chem. 269, 13254–13258 (1994).
Tomes, C.N., McMaster, C.R. & Saling, P.M. Activation of mouse sperm phosphatidylinositol-4,5 bisphosphate-phospholipase C by zona pellucida is modulated by tyrosine phosphorylation. Mol. Reprod. Dev. 43, 196–204 (1996).
Fukami, K. et al. Requirement of phospholipase Cδ4 for the zona pellucida-induced acrosome reaction. Science 292, 920–923 (2001).
Roldan, E.R.S., Murase, T. & Shi, Q.-X. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science 266, 1578–1581 (1994).
Florman, H.M., Tombes, R.M., First, N.L. & Babcock, D.F. An adhesion-associated agonist from the zona pellucida activates G protein-promoted elevations of internal Ca and pH that mediate mammalian sperm acrosomal exocytosis. Dev. Biol. 135, 133–146 (1989).
Arnoult, C., Zeng, Y., & Florman, H.M. ZP3-dependent activation of sperm cation channels regulates acrosomal secretion during mammalian fertilization. J. Cell Biol. 134, 637–645 (1996).
Florman, H.M. Sequential focal and global elevations of sperm intracellular Ca2+ are initiated by the zona pellucida during acrosomal exocytosis. Dev. Biol. 165, 152–164 (1994).
Minke, B. & Cook, B. TRP Channel Proteins and Signal Transduction. Physiol. Rev. 82, 429–472 (2002).
Wissenbach, U., Schroth, G., Phillipp, S. & Flockerzi, V. Structure and mRNA expression of a bovine trp homologue related to mammalian trp2 transcripts. FEBS Lett. 429, 61–66 (1998).
Jungnickel, M.K., Marrero, H., Birnbaumer, L., Lemos, J.R. & Florman, H.M. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nature Cell Biol. 5, 499–502 (2001).
Trevino, C.L., Serrano, C.J., Beltran, C., Felix, R. & Darszon, A. Identification of mouse trp homologs and lipid rafts from spermatogenic cells and sperm. FEBS Lett. 509, 119–125 (2001).
Walensky, L.D. & Snyder, S.H. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J. Cell Biol. 130, 857–869 (1995).
Wes, P.D. et al. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl Acad. Sci. USA 92, 9652–9656 (1995).
Vannier, B. et al. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ channel. Proc. Natl Acad. Sci. USA 96, 2060–2064 (1999).
Hutt, D.M., Cardullo, R.A., Baltz, J.M. & Kgsee, J.K. Synaptotagmin VIII Is localized to the mouse sperm head and may function in acrosomal exocytosis. Biol. Reprod. 66, 50–56 (2002).
Schulz, J.R., Wessel, G.M. & Vacquier, V.D. The exocytosis regulatory proteins syntaxin and VAMP are shed from sea urchin sperm during the acrosome reaction. Dev. Biol. 191, 80–87 (1997).
Michaut, M., Tomes, C.N., De Blas, G., Yunes, R. & Mayorga, L.S. Calcium-triggered acrosomal exocytosis in human spermatozoa requires the coordinated activation of Rab3A and N-ethylmaleimide-sensitive factor. Proc. Natl Acad. Sci. USA 97, 9996–10001 (2000).
Cummins, J.M. & Yanagimachi, R. Development of ability to penetrate the cumulus oophorus by hamster spermatozoa capacitated in vitro, in relation to the timing of the acrosome reaction. Gamete Res. 15, 187–212 (1986).
Evans, J.P. Sperm disintegrins, egg integrins, and other cell adhesion molecules of mammalian gamete plasma membrane interactions. Front. Biosci. 4, D114–D131 (1999).
Cuasnicu, P.S. et al. Molecular mechanisms involved in mammalian gamete fusion. Arch. Med. Res. 32, 614–618 (2001).
Cho, C. et al. Fertilization defects in sperm from mice lacking fertilin β. Science 281, 1857–1859 (1998).
Nishimura, H., Cho, C., Branciforte, D.R., Myles, D.G. & Primakoff, P. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin β. Dev. Biol. 233, 204–213 (2001).
Zhu, X., Bansal, N.P. & Evans, J.P. Identification of key functional amino acids of the mouse fertilin β (ADAM2) disintegrin loop for cell–cell adhesion during fertilization. J. Biol. Chem. 275, 7677–7683 (2000).
Bigler, D. et al. Sequence-specific interaction between the disintegrin domain of mouse ADAM 2 (fertilin β) and murine eggs. Role of the α6 integrin subunit. J. Biol. Chem. 275, 11576–11584 (2000).
Takahashi, Y., Bigler, D., Ito, Y. & White, J.M. Sequence-specific interaction between the disintegrin domain of mouse ADAM 3 and murine eggs: role of β1 integrin-associated proteins CD9, CD81, and CD98. Mol. Biol. Cell 12, 809–820 (2001).
Chen, M.S. et al. Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin α6β1: implications for murine fertilization. Proc. Natl Acad. Sci. USA 96, 11830–11835 (1999).
Almeida, E.A. et al. Mouse egg integrin α6β1 functions as a sperm receptor. Cell 81, 1095–1104 (1995).
Chen, H. & Sampson, N.S. Mediation of sperm–egg fusion: evidence that mouse egg α6β1 integrin is the receptor for sperm fertilin β. Chem. Biol. 6, 1–10 (1999).
Miller, B.J., Georges-Labouesse, E., Primakoff, P. & Myles, D.G. Normal fertilization occurs with eggs lacking the integrin α6β1 and is CD9-dependent. J. Cell Biol. 149, 1289–1296 (2000).
Evans, J.P. Fertilin β and other ADAMs as integrin ligands: insights into cell adhesion and fertilization. BioEssays 23, 628–639 (2001).
Zhu, X. & Evans, J.P. Analysis of the roles of RGD-binding integrins, α4/α9 integrins, α6 integrins, and CD9 in the interaction of the fertilin β (ADAM2) disintegrin domain with the mouse egg membrane. Biol. Reprod. 66, 1193–1202 (2002).
Eto, K. et al. Functional Classification of ADAMs Based on a Conserved Motif for Binding to Integrin α9β1. Implications for sperm–egg binding and other cell interactions. J. Biol. Chem. 277, 17804–17810 (2002).
Le Naour, F., Rubinstein, E., Jasmin, C., Prenant, M. & Boucheix, C. Severely reduced female fertility in CD9-deficient mice. Science 287, 319–321 (2000).
Miyado, K. et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 287, 321–324 (2000).
Kaji, K. et al. The gamete fusion process is defective in eggs of CD9-defective mice. Nature Genet. 24, 279–282 (2000).
Zhu, G.Z. et al. Residues SFQ (173–175) in the large extracellular loop of CD9 are required for gamete fusion. Development 129, 1995–2002 (2002).
Wong, G.E., Zhu, X., Prater, C.E., Oh, E. & Evans, J.P. Analysis of fertilin α (ADAM1)-mediated sperm-egg cell adhesion during fertilization and identification of an adhesion-mediating sequence in the disintegrin-like domain. J. Biol. Chem. 276, 24937–24945 (2001).
Hemler, M.E. Specific tetraspanin functions. J. Cell Biol. 155, 1103–1107 (2001).
Boucheix, C. & Rubinstein, E. Tetraspanins. Cell Mol. Life Sci. 58, 1189–1205 (2001).
Blobel, C.P. et al. A potential fusion peptide and an integrin ligand domain in a protein active in sperm–egg fusion. Nature 356, 248–252 (1992).
Shamsadin, R. et al. Male mice deficient for germ-cell cyritestin are infertile. Biol. Reprod. 61, 1445–1451 (1999).
Ulrich, A.S., Otter, M., Glabe, C.G. & Hoekstra, D. Membrane fusion is induced by a distinct peptide sequence of the sea urchin fertilization protein bindin. J. Biol. Chem. 273, 16748–16755 (1998).
Kresge, N., Vacquier, V.D. & Stout, C.D. The crystal structure of a fusagenic sperm protein reveals extreme surface properties. Biochemistry 40, 5407–5413 (2001).
Runft, L.L., Jaffe, L.A. & Mehlmann, L.M. Egg activation at fertilization: where it all begins. Dev. Biol. 245, 237–254 (2002).
Stricker, S.A. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 211, 157–176 (1999).
Jones, K.T., Carroll, J., Merriman, J.A., Whittingham, D.G. & Kono, T. Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development 121, 3259–3266 (1995).
Schultz, R.M. & Kopf, G.S. Molecular basis of mammalian egg activation. Curr. Top. Dev. Biol. 30, 21–62 (1995).
Kline, D. & Kline, J.T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev. Biol. 145, 80–89 (1992).
Giusti, A.F., Carroll, D.J., Abassi, Y.A. & Foltz, K.R. Evidence that a starfish egg Src family tyrosine kinase associates with PLC-γ1 SH2 domains at fertilization. Dev. Biol. 208, 189–199 (1999).
Giusti, A.F. et al. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J. Biol. Chem. 274, 29318–29322 (1999).
Giusti, A.F., Xu, W., Hinkle, B., Terasaki, M. & Jaffe, L.A. Evidence that fertilization activates starfish eggs by sequential activation of a Src-like kinase and phospholipase Cγ. J. Biol. Chem. 275, 16788–16794 (2000).
Kinsey, W.H. & Shen, S.S. Role of the Fyn kinase in calcium release during fertilization of the sea urchin egg. Dev. Biol. 225, 253–264 (2000).
Runft, L.L. & Jaffe, L.A. Sperm extract injection into ascidian eggs signals Ca2+ release by the same pathway as fertilization. Development 127, 3227–3236 (2000).
Carroll, D.J. et al. Calcium release at fertilization in starfish eggs is mediated by phospholipase Cγ. J. Cell Biol. 138, 1303–1311 (1997).
Carroll, D.J., Albay, D.T., Terasaki, M., Jaffe, L.A. & Foltz, K.R. Identification of PLCγ-dependent and -independent events during fertilization of sea urchin eggs. Dev.Biol. 206, 232–247 (1999).
Mehlmann, L.M., Carpenter, G., Rhee, S.G. & Jaffe, L.A. SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev. Biol. 203, 221–232 (1998).
Mehlmann, L.M., Chattopadhyay, A., Carpenter, G., & Jaffe, L.A. Evidence that phospholipase C from the sperm is not responsible for initiating Ca2+ release at fertilization in mouse eggs. Dev. Biol. 236, 492–501 (2001).
Moore, G.D., Ayabe, T., Visconti, P.E., Schultz, R.M. & Kopf, G.S. Roles of heterotrimeric and monomeric G proteins in sperm-induced activation of mouse eggs. Development 120, 3313–3323 (1994).
Williams, C.J., Mehlmann, L.M., Jaffe, L.A., Kopf, G.S. & Schultz, R.M. Evidence that Gq family G proteins do not function in mouse egg activation at fertilization. Dev. Biol. 198, 116–127 (1998).
Campbell, K.D., Reed, W.A. & White, K.L. Ability of integrins to mediate fertilization, intracellular calcium release, and parthenogenetic development in bovine oocytes. Biol. Reprod. 62, 1702–1709 (2000).
Iwao, Y. & Fujimura, T. Activation of Xenopus eggs by RGD-containing peptides accompanied by intracellular Ca2+ release. Dev. Biol. 177, 558–567 (1996).
Shilling, F.M., Magie, C.R. & Nuccitelli, R. Voltage-dependent activation of frog eggs by a sperm surface disintegrin peptide. Dev. Biol. 202, 113–124 (1998).
Tesarik, J. & Mendoza, C. In vitro fertilization by intracytoplasmic sperm injection. BioEssays 21, 791–801 (1999).
Kuretake, S., Kimura, Y., Hoshi, K. & Yanagimachi, R. Fertilization and development of mouse oocytes injected with isolated sperm heads. Biol.Reprod. 55, 789–795 (1996).
Kimura, Y. et al. Analysis of mouse oocyte activation suggests the involvement of sperm perinuclear material. Biol.Reprod. 58, 1407–1415 (1998).
Parrington, J., Swann, K., Shevchenko, V.I., Sesay, A.K. & Lai, F.A. Calcium oscillations in mammalian eggs triggered by a soluble sperm protein. Nature 379, 364–368 (1996).
Sette, C., Bevilacqua, A., Geremia, R., & Rossi, P. Involvement of phospholipase Cγ1 in mouse egg activation induced by a truncated form of the C-kit tyrosine kinase present in spermatozoa. J. Cell Biol. 142, 1063–1074 (1998).
Wolosker, H. et al. Molecularly cloned mammalian glucosamine-6-phosphate deaminase localizes to transporting epithelium and lacks oscillin activity. FASEB J. 12, 91–99 (1998).
Wolny, Y.M. et al. Human glucosamine-6-phosphate isomerase, a homologue of hamster oscillin, does not appear to be involved in Ca2+ release in mammalian oocytes. Mol. Reprod. Dev. 52, 277–287 (1999).
Kuo, R.C. et al. NO is necessary and sufficient for egg activation at fertilization. Nature 406, 633–636 (2000).
Hyslop, L.A., Carroll, M., Nixon, V.L., McDougall, A. & Jones, K.T. Simultaneous measurement of intracellular nitric oxide and free calcium levels in chordate eggs demonstrates that nitric oxide has no role at fertilization. Dev. Biol. 234, 216–230 (2001).
Rice, A., Parrington, J., Jones, K.T. & Swann, K. Mammalian sperm contain a Ca2+-sensitive phospholipase C activity that can generate InsP(3) from PIP(2) associated with intracellular organelles. Dev. Biol. 228, 125–135 (2000).
Heyers, S. et al. Activation of mouse oocytes requires multiple sperm factors but not sperm PLCγ1. Mol. Cell Endocrinol. 166, 51–57 (2000).
Wu, H. et al. Sperm factor induces intracellular free calcium oscillations by stimulating the phosphoinositide pathway. Biol. Reprod. 64, 1338–1349 (2001).
Acknowledgements
We would like to thank the members of our laboratories for valuable discussions and the National Institutes of Health for supporting our efforts.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Evans, J., Florman, H. The state of the union: the cell biology of fertilization. Nat Med 8 (Suppl 10), S57–S63 (2002). https://doi.org/10.1038/nm-fertilityS57
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm-fertilityS57
This article is cited by
-
A Model for the Acrosome Reaction in Mammalian Sperm
Bulletin of Mathematical Biology (2018)