Abstract
Purpose
To compare optical coherence tomographic angiography (OCTA) and indocyanine green angiography (ICGA) images for detecting polypoidal lesions (PLs) and branching vascular networks (BVNs), and to measure the polypoidal areas (PAs) in patients with polypoidal choroidal vasculopathy (PCV).
Methods
All patients underwent ICGA, optical coherence tomography (OCT), and OCTA. We compared the detection sensitivity for PL and BVN, as evaluated by the ICGA and OCTA images. Furthermore, PA measured by ICGA was divided into two groups: one in which the area could be measured by OCTA (ICGA+OCTA+) and the other in which the area could not be measured by OCTA (ICGA+OCTA−).
Results
Twenty-one consecutive eyes of 21 patients (mean age, 73.8±9.8 years) were included. ICGA detected PL in all eyes (100%), whereas OCTA detected PL in 16 eyes (75.2%); ICGA detected BVN in 15 eyes (71.4%), whereas OCTA detected BVN in 20 eyes (95.2%). The mean PA in ICGA+OCTA+ and ICGA+OCTA− was 0.24±0.04 and 0.14±0.01 mm2, respectively; a significant difference was observed between ICGA+OCTA+ PA and ICGA+OCTA− PA (P<0.0001). In addition, the mean PA in the ICGA+OCTA+ group measured by ICGA and OCTA was 0.24±0.04 was 0.19±0.04 mm2, respectively; these values were significantly different (P=0.0046).
Conclusions
OCTA might detect more BVNs and fewer PLs compared with ICGA, and PL detected by OCTA might be smaller than those detected by ICGA.
Similar content being viewed by others
Introduction
Polypoidal choroidal vasculopathy (PCV) involves choroidal neovascularisation (CNV) characterised by polypoidal lesions (PLs) with or without branching vascular networks (BVNs) under the retinal pigment epithelium (RPE).1, 2, 3 Microscopic fundus examination, optical coherence tomography (OCT), fluorescein angiography (FA), and indocyanine green angiography (ICGA) are useful for diagnosing eyes with PCV.4, 5, 6, 7, 8, 9, 10, 11 FA and ICGA are invasive techniques that require intravenous dye injection, and hepatic, renal, or allergic complications can arise.12 In terms of severe reactions, cases of hypotensive shock and death have been reported.13 Therefore, patients with a history of allergies or hepatic or renal dysfunction are not suited for angiographic examination.
New imaging technologies have recently been developed, which provide new insights into the visualisation of normal and pathologic vascularisation. The development of spectral-domain OCT instruments with increased scanning speeds and high-speed swept-source OCT instruments has enabled the use of optical coherence tomographic angiography (OCTA).14, 15, 16, 17 OCTA, which can noninvasively detect blood flow, provide three-dimensional reconstruction of the perfused microvasculature within the retina and choroid, and identify distinct characteristics of the capillary networks located within the different layers of the retina and choroid; thus, it is a useful tool for diagnosing many ocular diseases. Images of PL and BVN in eyes with PCV, and the detection abilities of OCTA and ICGA for detecting PCV have been compared;18 however, to the best of our knowledge, the accuracy of OCTA and ICGA in assessing PL has not yet been reported. The purpose of this study was to compare the accuracy of OCTA and ICGA images for detecting PL and BVN, and for measuring the polypoidal area (PA) in PCV.
Materials and methods
Patients
This study was approved by the Institutional Review Board of the Nagoya University Graduate School of Medicine. Informed consent was obtained from all patients, and all research and data collection complied with the tenets of the Declaration of Helsinki. We enrolled consecutive 21 cases of eyes with naive PCV seen at our department between March 2015 and August 2015. PCV was diagnosed based on early subretinal ICGA hyperfluorescence (appearing in a 3 × 3 mm area centred on the fovea within the first 5 min of dye injection) and at least one of the following diagnostic criteria according to the EVEREST study.19 All patients underwent a complete ophthalmic examination including best-corrected visual acuity (BCVA), anterior segment examination and dilated fundus biomicroscopy, colour fundus photos by CF-60 DSi (Canon, Tokyo, Japan), FA and ICGA by HRA II (Heidelberg Engineering, Heidelberg, Germany), and OCT image by Heidelberg Spectralis (Heidelberg, Germany). Patients who had eye of >25 and <22 mm axial length, PL outside of the scanning area, any other confounding retinal pathology such as diabetic retinopathy or pathologic myopia, previously had been treated with photodynamic therapy, intravitreal injection therapy, transpupil thermal laser, vitrectomy, or previously had any corneal disease or cataract making any images unclear, were excluded from the study.
Optical coherence tomographic microangiography
OCTA was measured by RTvue XR Avanti (Optovue Inc., Fremont, CA, USA) and was used to obtain OCT angiograms as previously described.16 This instrument has an A-scan rate of 70 000 scans per second using a light source centred at 840 nm and a bandwidth of 50 nm; the resolution is 5 mm axially, with a 15 mm beam width. Each B scan contained 316 A-scans, and two consecutive B-scans (M-B frames) were captured at a fixed position before proceeding to the next sampling location. The volumes were registered and the B-scan images were compared to calculate the decorrelation in the images; the decorrelation was viewed as the maximal projection image of blood flow. Because the retina is a laminar structure with a corresponding stratification of blood supply, segmentation of the retina in specific layers allows simple en face visualisation of the corresponding vascular supply in that layer.
Evaluation of PA
We investigated how PA detected by ICGA could be depicted by OCTA, and we measured PA from the paired images. To measure PA from ICGA, we used ICGA images at about 60 s after intravenous dye injection. We traced the border of the area that included the hyperfluorescent lesion (representing PL) in the ICGA image and measured the pixels of PL using NAVIS bundled software (Nidek Co. Ltd., Aichi, Japan). The area of one pixel in the ICGA images was calculated as 0.0004 mm2, and all areas were converted from pixels to the area (mm2). We used 3 × 3 mm2 OCTA images in the outer retinal layer to measure PA by OCTA. We traced the border between the areas containing the abnormal capillaries in the same position as the hyperfluorescent lesion on the ICGA images, and measured the pixels using the same method. The area of one pixel in the OCTA images was calculated as 0.000075 mm2, and all areas were converted from pixels to the area (mm2). The detection of PL and BVN in the paired images and measures of PA were performed by two observers (KT and KK), who were both masked to the patients’ clinical status.
Outcomes and statistical analyses
The detection rates of PL and BVN, and the PA were compared. The area data are shown as the mean±SEM. The one-sided Wilcoxon signed-rank test was used to analyse the outcomes statistically. A probability of P<0.05 was considered statistically significant.
Results
Patient characteristics
Twenty-one eyes of 21 patients (15 males and 6 females, all Japanese, Table 1) with PCV were included in the study. They ranged in age from 51 to 90 years, with a mean age of 73.8±9.8 years. The BCVAs ranged from 3/20 to 25/20. The biomicroscopic fundus examinations revealed an orange-red lesion and/or subretinal haemorrhage. OCT showed highly reflective RPE detachments, serous retinal detachment, or macular oedema at the posterior pole, particularly in the macular region in all patients.
Sensitivity of PL and BVN detection by ICGA and OCTA
Detection of PL and BVN by ICGA and OCTA were shown in Table 1. Both ICGA and OCTA detected both PL and BVN in 10 eyes (Case # 1–10). ICGA detected only PL but not BVN, whereas OCTA detected both PL and BVN in five eyes (Case # 11–15). ICGA detected both PL and BVN, whereas OCTA detected only BVN but not PL in five eyes (Case # 16–20). Both ICGA and OCTA detected PL but not BVN in one eye (Case # 21).
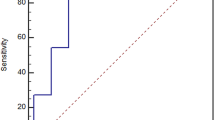
The eye in which both ICGA and OCTA detected PL and BVN was from Case 1; the BCVA was 3/20 and the PAs were 0.49 mm2 in ICGA and 0.46 mm2 in OCTA (Figure 1). The eye in which ICGA detected only PL, although OCTA detected both of PL and BVN, was from Case 11. The BCVA was 12/20, and the PAs were 0.20 mm2 in ICGA and 0.19 mm2 in OCTA (Figure 2). In Case 16, ICGA detected both of PL and BVN, but OCTA detected only BVN. The BCVA was 10/20 and the PA was 0.20 in ICGA (Figure 3). The detection percentages of PL and BVN in ICGA and OCTA are shown in Figure 4a. ICGA detected PL in all eyes (100%) and BVN in 15 eyes (71.4%), whereas OCTA detected PL in 16 eyes (76.2%) and BVN in 20 eyes (95.2%).
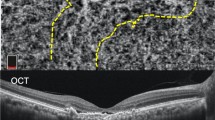
Case 11, in which ICGA could detect only PL, although OCTA detected PL and BVN. Colour fundus (a), OCT image (b), ICGA image (c), and OCTA image (d). White arrow: PL. Black arrow: BVN. In the ICGA image, BVN was unclear, although it appeared clearly in the OCTA images. The PAs were 0.20 mm2 in ICGA and 0.19 mm2 in OCTA.
Case 16, in which ICGA, but not OCTA, detected PL. Colour fundus (a), OCT image (b), ICGA image (c), and OCTA image (d). White arrow: PL. Black arrow: BVN. BVN was detected by ICGA and OCTA, and was observed with highly bright vascularity, although PL was shown in dark spot in OCTA. The PA was 0.20 mm2 in ICGA.
Detection percentages of PL and BVN, and the differences between the PA detected by OCTA and not detected by OCTA, and the PA measured by ICGA and by OCTA. ICGA detected PL more often than ICGA, although ICGA detected BVN less often than OCTA. (a) The mean PAs measured from the ICGA images in all eyes, eyes detected by OCTA (ICGA+OCTA+), and eyes that were not detected by OCTA (ICGA+OCTA−). (b) The mean PA measured from the ICGA and OCTA images in 15 eyes. (c) The blots of PA of each eye in ICGA and OCTA (d).
PA in ICGA and OCTA
We compared the differences in PA size between the cases detected by both ICGA and OCTA (ICGA+OCTA+), and those detected by ICGA, but not by OCTA (ICGA+OCTA−). Figure 4b shows the mean PA as measured by the ICGA images in all eyes, as well as that in ICGA+OCTA+ and ICGA+OCTA−. The mean PAs were 0.21±0.03 mm2 in all eyes, 0.24±0.04 mm2 in ICGA+OCTA+ eyes, and 0.14±0.01 mm2 in ICGA+OCTA− eyes. Significant differences were found between the mean PA in ICGA+OCTA+ and ICGA+OCTA− (P<0.001). We also compared the values of PA in ICGA+OCTA+ as measured by both ICGA and OCTA. Figure 4c shows the mean PA measured from the ICGA and OCTA images in all 16 eyes. The mean PA was 0.24±0.04 mm2 in ICGA and 0.19±0.04 mm2 in OCTA. The mean PA measured by ICGA was significantly larger than that measured by OCTA (P=0.0046). Figure 4d shows the correlation of the PA in each eye measured by both ICGA and OCTA. Of 16 eyes, the PA in 14 eyes (87.5%) was smaller in OCTA than in ICGA, and the PA in two eyes (12.5%) was larger than in ICGA.
Discussion
This study showed that OCTA could detect PL and BVN, and that its detection sensitivity was lower for PL and higher for BVN than detection by ICGA. Smaller areas of PL were not detected by OCTA, and the detected areas were smaller than those detected by ICGA. OCTA acquires images by detecting the motion of scattering particles, such as erythrocytes, within sequential OCT cross-sectional scans performed repeatedly at the same location.20 The temporal changes in the OCT signal caused by erythrocytes generate contrast and allow visualisation of the microvasculature. In PL, CNV are filled in the polyp, and the vessel was curved three-dimensionally.21 Srour et al18 reported that OCTA could be used to detect hyperflow structure of only 25% of PL. In this study, smaller values of PL could not be detected by OCTA. The reason for this could be that smaller values of PL correspond to small or sharply curved vessels in which it is more difficult for erythrocytes to move. OCTA could not detect erythrocyte motion; therefore, smaller values of PL could not be detected. On the other hand, PAs detected by OCTA were smaller than those detected by ICGA in 14 of 16 eyes and the mean PA measured from the OCTA images was significantly smaller than that from the ICGA images. Two assessments might be considered to explain the smaller detection. First, we measured the area from the ICGA image around 60 s after intravenous dye injection. PL consisted of the hyalinisation of choroidal vessels, and massive exudation of fibrin and blood plasma.22 The dye leaked to the outside of choroidal vessels, and we measured the area as PL. Second, the temporal changes in the OCT signal caused by the erythrocytes generate contrast and allow visualisation of the inside of the microvasculature. The microvasculature shows hyalinisation of the choroidal vessels like in arteriosclerosis, and the vessel walls are thick.3 OCTA could detect the inner diameter of CNV from the motion of the erythrocytes, which was smaller than the outer diameter of the vessels.
Tan et al19 reported that ICGA detected BVN in 77.6% of eyes with PCV. In the present study, all of the eyes exhibited PL, and 15 eyes (71.4%) exhibited BVN as measured by ICGA, which is a similar rate to that from the previous reports.5,9, 20,22,23 However, OCTA detected BVN in 20 eyes (95.2%). This result suggests that eyes with PCV would have a higher BVN than BVN found in the previous ICGA reports; OCTA has a better ability to detect these networks than ICGA.
OCTA is reportedly useful for detecting many diseases,17, 23, 24 including age-related macular degeneration.14, 15 When compared with the ICGA images, the OCTA images are comparable to, if not better than, the ICGA images in terms of the ability to visualise PL, although they present several limitations. Kim et al compared the detection percentages of PL and BVN in OCTA and ICGA, and found that OCTA could detect BVN in all eyes, although it only detected half of the PLs. In particular, it requires that the patient fixate on a precise point for several seconds, and the images are restricted to a small area (3 × 3 or 6 × 6 mm). Despite these limitations, OCTA has several distinct advantages over ICGA imaging, such as its noninvasiveness, speed, its ability to detect details of the retinal and choroidal vasculature without intravenous dye injection, and its ability to measure blood flow. Moreover, it is an evolving technique, and the interpretation of the images needs to be refined further.
In conclusion, this study showed OCTA images of eyes with PCV. With this imaging technique, we were able to demonstrate the presence of PL and BVN that was detectable by ICGA. Despite the relatively small number of eyes included in this study, the OCTA findings were consistent and repeatable. Further investigations in larger groups of subjects with PCV are needed to assess the definitive role of this new imaging modality. OCTA might be a useful tool to noninvasively diagnose and maintain eyes with PCV.

References
Yannuzzi LA, Wong DW, Sforzolini BS, Goldbaum M, Tang KC, Spaide RF et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol 1999; 117: 1503–1510.
Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 2003; 121: 1392–1396.
Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA . Polypoidal choroidal vasculopathy. Surv Ophthalmol 2004; 49: 25–37.
Schachat AP, Thompson JT . Optical coherence tomography, fluorescein angiography, and the management of neovascular age-related macular degeneration. Ophthalmology 2015; 122: 222–223.
Kokame GT . Prospective evaluation of subretinal vessel location in polypoidal choroidal vasculopathy (PCV) and response of hemorrhagic and exudative PCV to high-dose antiangiogenic therapy (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2014; 112: 74–93.
De Salvo G, Vaz-Pereira S, Keane PA, Tufail A, Liew G . Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 2014; 158: 1228–1238 e1221.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA . Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995; 15: 100–110.
Wilde C, Patel M, Lakshmanan A, Amankwah R, Dhar-Munshi S, Amoaku W . The diagnostic accuracy of spectral-domain optical coherence tomography for neovascular age-related macular degeneration: a comparison with fundus fluorescein angiography. Eye 2015; 29: 602–609.
Liu R, Li J, Li Z, Yu S, Yang Y, Yan H et al. Distinguishing polypoidal choroidal vasculopathy from typical neovascular age-related macular degeneration based on spectral domain optical coherence tomography. Retina 2016; 36: 778–786.
Kokame GT, Hirai K, Yanagihara R . Polypoidal choroidal vasculopathy: imaging by indocyanine green angiography and en face optical coherence tomography. JAMA Ophthalmol 2015; 133: e151886.
Ting DS, Cheung GC, Lim LS, Yeo IY . Comparison of swept source optical coherence tomography and spectral domain optical coherence tomography in polypoidal choroidal vasculopathy. Clin Exp Ophthalmol 2015; 43: 815–819.
Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W et al. Fluorescein angiography complication survey. Ophthalmology 1986; 93: 611–617.
Bonte CA, Ceuppens J, Leys AM . Hypotensive shock as a complication of infracyanine green injection. Retina 1998; 18: 476–477.
de Carlo TE, Bonini Filho MA, Chin AT, Adhi M, Ferrara D, Baumal CR et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology 2015; 122: 1228–1238.
Kuehlewein L, Sadda SR, Sarraf D . OCT angiography and sequential quantitative analysis of type 2 neovascularization after ranibizumab therapy. Eye 2015; 29: 932–935.
Spaide RF . Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am J Ophthalmol 2015; 160: 6–16.
Ang M, Sim DA, Keane PA, Sng CC, Egan CA, Tufail A et al. Optical coherence tomography angiography for anterior segment vasculature imaging. Ophthalmology 2015; 122: 1740–1747.
Srour M, Querques G, Semoun O, El Ameen A, Miere A, Sikorav A et al. Optical coherence tomography angiography characteristics of polypoidal choroidal vasculopathy. Br J Ophthalmol 2016. e-pub ahead of print 2 February 2016; doi:10.1136/bjophthalmol-2015-307892.
Tan CS, Ngo WK, Chen JP, Tan NW, Lim TH . EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br J Ophthalmol 2015; 99: 624–628.
Choi W, Mohler KJ, Potsaid B, Lu CD, Liu JJ, Jayaraman V et al. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS One 2013; 8: e81499.
Yuzawa M, Mori R, Kawamura A . The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol 2005; 89: 602–607.
Lim TH, Laude A, Tan CS . Polypoidal choroidal vasculopathy: an angiographic discussion. Eye 2010; 24: 483–490.
Christenbury JG, Klufas MA, Sauer TC, Sarraf D . OCT angiography of paracentral acute middle maculopathy associated with central retinal artery occlusion and deep capillary ischemia. Ophthalmic Surg Lasers Imaging Retina 2015; 46: 579–581.
McClintic SM, Jia Y, Huang D, Bailey ST . Optical coherence tomographic angiography of choroidal neovascularization associated with central serous chorioretinopathy. JAMA Ophthalmol 2015; 133: 1212–1214.
Acknowledgements
We express the deepest appreciation to all patients included in the study and all staff working in my department. This research was supported by a Grant-in-Aid for Scientific Research B (No. 23390401[SU]) and a Grant-in-Aid for Scientific Research C (No. 25462710) from the Ministry of Education, Culture, Sports, Science and Technology (http://www.jsps.go.jp/). HT has research funding from Novartis Pharma KK Novartis Co.; Santen Pharmaceutical Co.; Wakamoto Co.; Pfizer Inc. Co., and personal fees from Bayer Healthcare AG Co.; Novartis Pharma KK Co.; Santen Pharmaceutical Co.; Wakamoto Co.; and Pfizer Inc. Co.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Takayama, K., Ito, Y., Kaneko, H. et al. Comparison of indocyanine green angiography and optical coherence tomographic angiography in polypoidal choroidal vasculopathy. Eye 31, 45–52 (2017). https://doi.org/10.1038/eye.2016.232
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.232
This article is cited by
-
Predicting treatment outcomes of intravitreal brolucizumab for polypoidal choroidal vasculopathy through noninvasive assessment of polypoidal lesion blood flow with optical coherence tomography angiography
Scientific Reports (2024)
-
Small dome-shaped pigment epithelium detachment in polypoidal choroidal vasculopathy: an under-recognized sign of polypoidal lesions on optical coherence tomography?
Eye (2022)
-
Clinical features and prognosis of polypoidal choroidal vasculopathy with different morphologies of branching vascular network on optical coherence tomography angiography
Scientific Reports (2021)
-
Characterizing Branching Vascular Network Morphology in Polypoidal Choroidal Vasculopathy by Optical Coherence Tomography Angiography
Scientific Reports (2019)
-
Comparison between non-visualized polyps and visualized polyps on optical coherence tomography angiography in polypoidal choroidal vasculopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2019)