Abstract
Purpose
To report the clinical manifestations and treatment outcomes of patients with presumed intraocular tuberculosis (TB) seen at the Newcastle Uveitis Service, Royal Victoria Infirmary, Newcastle upon Tyne, UK over a 10-year period.
Methods
Retrospective review of case notes.
Results
A total of 21 patients were identified. Occlusive retinal vasculitis was the commonest ophthalmological presentation (12 patients). Eight patients (38%) were found to have underlying active systemic TB (four with mediastinal lymphadenopathy, three with pulmonary TB, one with cutaneous TB). Constitutional or respiratory symptoms, elevated inflammatory markers, and an abnormal chest radiograph were poor indicators of active TB. Two patients had inactive intrathoracic TB. Eleven patients had latent TB. Eighteen patients received anti-tuberculous treatment. Final visual acuity was better than or equal to initial visual acuity in 14 out of 16 patients who completed at least 6 months of standard anti-tuberculous treatment.
Conclusions
Most patients with presumed intraocular TB have latent TB, but a significant minority has hitherto undetected active TB. Our series suggests that either proven or presumed intraocular TB occurs frequently in the absence of constitutional or respiratory symptoms, elevated inflammatory markers, or an abnormal chest radiograph. A minimum of 6 months standard anti-tuberculous treatment provides good visual outcomes in the majority of patients.
Similar content being viewed by others
Introduction
Tuberculosis (TB) is the world’s leading infectious cause of death. A third of the world’s population is infected with Mycobacterium tuberculosis (MTb), giving rise to over 9 million new cases of active disease and nearly 2 million deaths each year.1, 2 In the United Kingdom, there has been a gradual rise in the number of cases of active TB over the past 20 years. The current overall UK incidence is 15 new cases per 100 000 population per year, but there are much higher rates in major cities. For example in 2009, 3440 of the total 9040 cases in the United Kingdom (38%) occurred in London, giving a rate of 44.4 per 100 000. This compares with 169 new cases from the North East of England in the same year (1.9% of the United Kingdom total), giving a rate of 6.5 per 100 000, but a higher rate in Newcastle upon Tyne of 16 per 100 000.3
Extrapulmonary TB accounts for 46% of UK TB, affecting lymph nodes, bones, brain, gastro-intestinal tract, and other organs.3 However, intraocular TB is rare, manifesting as uveitis in a variety of clinical patterns. Intraocular TB has not been separately quantified in surveillance data from the UK Health Protection Agency or the World Health Organization, and there have been very few case series of intraocular TB from UK centres.4, 5, 6 We present the clinical manifestations and treatment outcomes of patients with presumed intraocular TB, seen in the Newcastle Uveitis Service over a 10-year period.
Materials and methods
Patient details were taken from the Newcastle Uveitis Service database for the period 1 January 2002 to 31 December 2011. Patients were diagnosed as having presumed intraocular TB if uveitis was accompanied by evidence of active or latent TB, no other cause or association of uveitis was identified after appropriate investigations, and the clinical pattern of uveitis did not clearly indicate a defined non-infectious isolated uveitis syndrome. Latent TB was diagnosed on the basis of a positive interferon-γ release assay (IGRA), in our series the Quanti-FERON TB Gold (QFT-G) test, or a positive tuberculin skin test (TST). The following data were retrospectively extracted from clinical records for all patients: age; sex; country of birth; ethnic origin; description of uveitis; history of previous TB or TB contact; presence of HIV or other immunodeficiency state; presence of constitutional, respiratory or other systemic symptoms; presence of abnormal physical signs; level of inflammatory markers (erythrocyte sedimentation rate, ESR and C-reactive protein, CRP); QFT-G result or TST result; chest radiograph and chest computed tomography (CT) findings; evidence of intrathoracic or extrathoracic TB; results of microscopy examination for acid-fast bacilli (AFBs); results of MTb culture; detection of MTb deoxyribonucleic acid by polymerase chain reaction (MTb-PCR); histology findings of biopsied tissues; details of treatment given, and the duration of follow-up. Best-corrected visual acuity was assessed at the start and end of the follow-up period in all patients.
Results
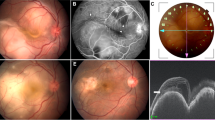
Twenty-one patients with presumed intraocular TB were identified (Tables 1 and 2). This group comprises 1.5% of the total 1360 patients with uveitis seen in the Newcastle Uveitis Service during the same period. Fifteen patients were male (71%). Mean age at presentation was 46 years (median 41 years; range 21–82 years). All patients were UK residents, of whom nine were born in the UK, six in India, four in Pakistan, one in Sudan, and one in Nigeria. In all, 11 patients were Asian (52%), 8 were White Caucasian (32%), and 2 were African (10%). There were no HIV-positive or otherwise immunocompromised patients in this series. Bilateral intraocular inflammation was present in 15 patients (71%). Twelve patients presented with occlusive retinal venous vasculitis accompanied by a variable degree of vitritis (57%) (Figure 1), two with panuveitis with multiple choroidal infiltrates (10%), two with large solitary choroidal granulomas (10%) (Figure 2), two with a multifocal choroiditis in the absence of vitritis (10%), two with chronic granulomatous anterior uveitis (10%), and one with a serpiginous-like choroiditis (5%) (Figure 3).
A history of previous pulmonary TB was present in one patient, who had received anti-tuberculous treatment (ATT) 5 years previously. No patient had a history of previous extrapulmonary TB. Five patients (24%) had a history of TB contact, but none of these had received anti-TB prophylaxis. Fifteen patients (71%) gave no history of previous TB or TB contact. Twenty patients underwent QFT-G testing, which was positive in all of them. In one patient (patient 13), the diagnosis of latent TB was made on the basis of strongly positive TST (Heaf Test Grade IV).
Eight patients (38%) were found to have underlying active systemic TB (four with mediastinal lymphadenopathy alone, three with pulmonary TB, and one with cutaneous TB). In all of these, the ophthalmological presentation was the first clinical manifestation of underlying active TB. Two patients (10%) had inactive intrathoracic TB (calcified mediastinal lymph nodes, lung scars/volume loss). Eleven patients (52%) had evidence of latent TB only.
Constitutional symptoms (appetite loss, weight loss, lethargy, fever, sweats, shivers) were present in two patients (10%), both of whom were subsequently found to have active intrathoracic TB (patients 8 and 11). None of our patients had respiratory symptoms or signs. Elevated ESR (>20 mm/h) and CRP (>5 mg/l) were found in only the two patients who had constitutional symptoms. A chest radiograph was performed in all patients and was normal in 15 patients (71%). Abnormal findings were mediastinal lymphadenopathy (four patients), apical fibrosis and volume loss suggestive of previous pulmonary TB (one patient), and pleural plaques from asbestos-related occupational exposure (one patient). Chest CT was performed in 17 patients and was abnormal in 10 patients. Abnormal findings were mediastinal lymphadenopathy alone (four patients), mediastinal lymphadenopathy and pulmonary nodules or cavitating lesions (three patients), calcified mediastinal lymph nodes with lung scars/volume loss consistent with inactive old pulmonary TB (two patients), and pleural plaques from asbestos exposure (one patient).
Of the eight patients with evidence of active systemic TB, two had histological evidence to support this (mediastinal lymph node and skin histology showing caseating granulomatous inflammation, respectively). In two other patients, active TB was confirmed by positive culture for MTb (mediastinal lymph node biopsy and broncho-alveolar lavage respectively). No patients had AFBs identified in biopsied tissue, sputum, or urine. In four patients, the diagnosis of active TB was based on the radiological findings. Vitreous biopsy for microscopy for AFBs, MTb culture, and MTb-PCR was carried out in two patients and these tests were all negative in both patients (Table 2).
A total of 19 patients (90%) in this series received ATT. In 18 patients, a standard course of ATT for a minimum of 6 months was started after the diagnosis of presumed intraocular TB had been made. This consisted of four drugs (ethambutol, pyrazinamide, rifampicin, and isoniazid) for 2 months, and two drugs (rifampicin and isoniazid) for at least a further 4 months. One patient had already received ATT 5 years previously for pulmonary TB (duration of ATT unknown) and re-treatment was not advised because both the pulmonary and the ophthalmological disease appeared inactive (patient 16). In another two patients with latent TB, ATT was not advised after careful consideration of individual risk/benefit (patients 13 and 14). In the 18 patients who underwent ATT after the onset of uveitis, mean follow-up between commencement of ATT and last clinical examination was 16.4 months (median 13 months; range 3–44 months). Compliance was good in all of these patients and no serious side effects of ATT occurred. In all, 16 patients completed at least 6 months of ATT, and in 2 patients ATT is ongoing. None of these patients had signs of active uveitis at the time of last examination or had recurrence of uveitis following 6 months ATT. Final best-corrected visual acuity was better than or equal to initial visual acuity in 14 of 16 patients who completed 6 months ATT. Macular scarring due to sub-foveal choroidal neovascular membrane (CNV) and a large posterior pole granuloma were the causes of worse final visual acuity in two patients (patients 6 and 7, respectively).
Posterior sub-Tenon’s triamcinolone 40 mg injection was performed in six patients (29%): for cystoid macular edema in five patients and for marked unilateral vitritis in one patient. Systemic corticosteroid treatment was given to control severe intraocular inflammation in four patients (20%). Pars plana vitrectomy was performed in two patients: for a non-clearing vitreous hemorrhage in one patient and as a diagnostic procedure for suspected endogenous endophthalmitis in the other. Nine patients with occlusive retinal vasculitis underwent laser photocoagulation to ischemic retinal areas. The indication for laser photocoagulations was retinal neovascularization with or without vitreous haemmorhage. One patient received photodynamic therapy for a secondary extra-foveal CNV and intravitreal ranibizumab injections for secondary sub-foveal CNV (patient 6).
Discussion
The diagnosis of intraocular TB is presumptive rather than definitive in almost all patients. It is based upon the presence of ophthalmological features consistent with intraocular TB, the exclusion of other causes of uveitis, immunological evidence of TB infection (either positive IGRA or TST) with or without evidence of active TB elsewhere in the body, and a beneficial response to ATT.6, 7, 8, 9 Definitive proof of intraocular TB by the demonstration of AFBs or culture of MTb from ocular specimens is exceedingly rare.7, 10 Failure to demonstrate AFBs or negative cultures do not exclude the possibility of intraocular TB, as the yield of micro-organisms from ocular fluids might be very low. MTb-PCR and has been used for early diagnosis of intraocular TB.11, 12, 13 In one study, MTb-PCR was detected from aqueous specimens in 60% of patients with presumed intraocular TB.11 PCR testing has yet to be standardized and is not recommended for TB diagnosis in the United Kingdom, because of its poor predictive value due to false negative results.14 Moreover, the pathophysiology of intraocular TB remains controversial. Ocular manifestations are caused either by active intraocular infection or as result of an immunological reaction in the eye in the absence of direct infection.10, 15 In the latter situation, evidence of MTb from ocular tissues may be lacking. Furthermore, there are several risks of intraocular biopsy, including visual loss from cataract, retinal detachment, or infection. In our series, diagnostic vitreous biopsy was performed in two patients. In both patients, specimens were negative for AFBs, MTb culture, and MTb-PCR but regression of intraocular inflammation and of their associated intrathoracic TB occurred after ATT, and no recurrences of uveitis were seen after completion of ATT.
The majority of patients with presumed intraocular TB are reported to have latent rather than active TB.6, 8, 16 This was evident in our series, in which only eight patients had active TB. A history of previous TB (1/21), TB contact history (5/21), constitutional symptoms (2/21), chest symptoms (0/21), abnormal chest signs (0/21), elevated inflammatory markers (2/21), or an abnormal chest radiograph (6/21) were very poor indicators of TB infection, and their absence should not discourage this diagnosis. The diagnosis of active intrathoracic TB would have been missed in three out of seven patients if only a chest radiograph without additional chest CT had been performed (patients 2, 8, and 19).
The large variations in the eye findings make the diagnosis of intraocular TB difficult. In their series from India, Gupta et al17 compared the presence of specific ocular findings between patients with presumed intraocular TB and patients with non-tubercular uveitis.17 They found that if the presence of any one of broad-based posterior synechiae, retinal vasculitis with choroiditis, retinal vasculitis without choroiditis, and serpiginous-like choroiditis is used to diagnose intraocular TB, then only 14% of patients with intraocular TB are likely to be missed. In the same study, retinal vasculitis with choroiditis, retinal vasculitis without choroiditis, and serpiginous-like choroiditis were found to have high positive predictive values but low negative predictive values for the diagnosis of intraocular TB, making it difficult to rule out TB if these signs are absent. In our series, occlusive retinal venous vasculitis was the most common ophthalmological finding, present in 12 patients (57%). Similarly, in the series of Rosen et al,4 florid ischemic retinal vasculitis with a marked tendency to neovascularization was the most common ocular manifestation of intraocular TB and was observed in 9 out of 12 patients included in their study. We suggest that further evaluation for underlying systemic TB should be considered in all patients with occlusive retinal venous vasculitis in whom no other systemic association for this is present.
TSTs such as the Mantoux or Heaf tests have been used for detection of TB infection for many years. There is no specific amount of induration that confirms TB infection, nor does a negative result exclude TB.15 The predictive value of TSTs varies depending on the population prevalence of TB and local BCG vaccination policy. The test is susceptible to placement errors, reading errors, false negative results (in elderly, malnourished, or immunocompromised patients including patients on corticosteroids or immunosuppressive agents), and false positive results (prior BCG vaccination and cross-reactivity with environmental mycobacteria).14 More recently, IGRAs have become available that measure interferon-γ released by peripheral T lymphocytes after stimulation by MTb antigens. The newer versions of IGRAs, such as the QFT-G assay, use highly specific MTb antigens that are not shared with BCG vaccine strains or other species of Mycobacteria.7 The body of literature supporting the use of IGRAs in patients with presumed intraocular TB is growing.18, 19, 20, 21 In December 2005, the US Center for Disease Control and Prevention updated their guidelines on the QFT-G assay, recommending its use in place of TST for infection control surveillance and detection of TB infection.22 Whereas the IGRAs are very useful in detecting MTb infection, they cannot differentiate latent from active TB.19, 23 Therefore, the results should be interpreted in their specific clinical context. In our hospital, QFT- G assay has been available since 2005. Over the time period of the current study, 48 patients seen in the Newcastle Uveitis Service underwent this assay accounting for 3.5% of all patients. Of these, 20 patients had presumed intraocular TB and are included in this series. The remainder had either a negative result or a positive result that was not associated etiologically with their uveitis (eg, some patients with specific non-infectious uveitis syndromes in whom the test was performed before commencing major immunosuppressive therapies to identify the presence of latent TB). We do not recommend using the QFT- G assay as a ‘scattergun’ approach in the investigation of uveitis, but as a useful diagnostic tool in selected patients in whom intraocular TB is suspected based on the history and clinical findings.
In our series, patients received a standard course of ATT for a minimum of 6 months irrespective of the presence of active or latent TB once the diagnosis of presumed intraocular TB had been made. A favorable ophthalmological response to this approach has been reported by several authors.5, 6, 8, 24, 25 Sanghvi et al6 found that in two-thirds of patients with presumed intraocular TB without evidence of active systemic TB treated with full 6 months of ATT, the uveitis remained inactive six months after completion of therapy. Bansal et al found that treatment with a combination of ATT and corticosteroids reduced the risk of developing a recurrence of uveitis by approximately two-thirds compared with treatment with corticosteroids alone.8 ATT has well recognized ocular and systemic adverse effects. Paradoxical reactions in the eye such as worsening of the inflammation or existing lesions, or appearance of new lesions have also been described.26, 27 However, we feel that standard full 6 months ATT is generally justified to minimize the risk of ocular damage. In our series, intraocular inflammation resolved without recurrence in all patients treated in this way. Although corticosteroid or immunosuppressive therapy may cause a re-activation of systemic TB resulting in severe disseminated TB and even a panophthalmitis,4, 26 we suggest that the presence of certain sight-losing complications, such as cystoid macular edema, or severe intraocular inflammation, justifies the use of corticosteroid treatment under the coverage of ATT. The route of administration of corticosteroids may be topical, periocular, or systemic. In their series, Bansal et al8 used topical or periocular corticosteroids in patients with predominantly anterior or intermediate uveitis and systemic steroids in patients with predominantly posterior uveitis (multifocal choroiditis, choroidal granuloma, retinal vasculitis, serpiginous-like choroiditis) as well as some patients with intermediate uveitis. In our series, periocular corticosteroids were used in six patients under ATT coverage (five patients with cystoid macular edema and one patient with marked vitritis). All six patients responded favorably and no adverse effects were observed.
In summary, this is the first published series of presumed intraocular TB from the North East of England. TB-associated uveitis accounted for 1.5% of all uveitis cases seen in the Newcastle Uveitis Service, a proportion similar to that reported recently from Manchester.6 Occlusive retinal venous vasculitis was present in the majority of patients. Our series suggests that either proven or presumed intraocular TB occurs frequently in the absence of constitutional or respiratory symptoms, elevated inflammatory markers or an abnormal chest radiograph. In suspected cases, further evaluation including IGRA or TST, CT scan of the chest, and referral to a TB physician should be considered. Most patients with presumed intraocular TB have latent TB, but a significant minority has hitherto undetected active TB. Patients should be offered at least a standard full 6 months ATT because this leads to improvement of intraocular inflammation, treats active TB in those who have it, and reduces the risk of developing active TB in those who do not. Additional corticosteroid treatment should be considered to control severe intraocular inflammation or macular edema. Outcomes using this approach are very good in the majority of patients.

References
Lönnroth K, Castro KG, Chacaya JM, Chauhan LS, Floyd K, Glaziou P et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet 2010; 375: 1814–1829.
World Health Organization. Global Tuberculosis Control Report. World Health Organization: Geneva, 2010.
Health Protection Agency. Tuberculosis in the UK. Annual report on tuberculosis surveillance in the UK. Health Protection Agency: London, 2010.
Rosen PH, Spalton DJ, Graham EM . Intraocular tuberculosis. Eye 1990; 4: 486–492.
Varma D, Anand S, Reddy AR, Das A, Watson JP, Currie DC et al. Tuberculosis: an under-diagnosed aetiological agent in uveitis with an effective treatment. Eye 2006; 20: 1068–1073.
Sanghvi C, Bell C, Woodhead M, Hardy C, Jones N . Presumed tuberculous uveitis: diagnosis, management and outcome. Eye 2011; 25: 475–480.
Gupta V, Gupta A, Rao N . Intraocular TB: An update. Surv Ophthalmol 2007; 52: 561–587.
Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK . Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. Am J Ophthalmol 2008; 146: 772–779.
Gupta A, Gupta V . Tubercular posterior uveitis. Int Ophthalmol Clin 2005; 45: 71–88.
Biswas J, Madhavan HN, Gopal L, Badrinath SS . Intraocular tuberculosis: clinicopathologic study of five cases. Retina 1995; 15: 461–468.
Gupta V, Arora S, Gupta A, Ram J, Bambery P, Sehgal S . Management of presumed intraocular tuberculosis: possible role of the polymerase chain reaction. Acta Ophthalmol Scand 1998; 76: 679–682.
Arora SK, Gupta V, Gupta A, Bambery P, Kapoor GS, Sehgal S . Diagnostic efficacy of polymerase chain reaction in granulomatous uveitis. Tuber Lung Dis 1999; 79: 229–233.
Ortega-Larrocea G, Bobadilla-del-Valle M, Ponce-de-Leon A, Sifuentes- Osornio J . Nested polymerase chain reaction for Mycobacterium tuberculosis DNA detection in aqueous and vitreous of patients with uveitis. Arch Med Res 2003; 34: 116–119.
The National Collaborating Centre for Chronic Conditions. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Royal College of Physicians: London, 2006.
Bodaghi B, LeHoang P . Ocular tuberculosis. Curr Opin Ophthalmol 2000; 11: 443–448.
Abrams J, Schlaegel TF . The role of isoniazid therapeutic test in tubercular uveitis. Am J Ophthalmol 1982; 94: 511–515.
Gupta A, Bansal R, Gupta V, Sharma A, Bambery P . Ocular signs predictive of tubercular uveitis. Am J Ophthalmol 2010; 149: 562–570.
Itty S, Bakri SJ, Pulido JS . Initial results of Quanti-FERON TB Gold testing in patients with uveitis. Eye 2009; 23: 904–909.
Pai M, Kalantri S, Dheda K . New tools and emerging technologies for the diagnosis of tuberculosis: Part I. Latent tuberculosis. Expert Rev Mol Diagn 2006; 6: 413–422.
Ang M, Htoon HM, Chee SP . Diagnosis of tuberculosis uveitis: clinical application of an interferon-gamma release assay. Ophthalmology 2009; 116: 1391–1396.
Mackensen F, Becker MD, Wiehler U, Max R, Dalpke A, Zimmermann S . Quanti-FERON TB Gold: a new test strengthening long-suspected tuberculous involvement in serpiginous-like choroiditis. Am J Ophthalmol 2008; 146: 761–766.
Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A . Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the Quanti-FERON TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54 (RR-15): 49–55.
European Centre for Disease Prevention and Control Guidance. Use of interferon-gamma release assays in support of TB diagnosis. European Centre for Disease Prevention and Control Guidance: Stockholm, 2011.
Van Daele PL, Bakker M, van Hagen PM, Baarsma GS, Kuijpers RW . TB or not TB: treat to see. Med J Aust 2006; 185: 178–179.
Morimura Y, Okada AA, Kawahara S, Miyamoto Y, Kawai S, Hirakata A et al. Tuberculin skin testing in uveitis patients and treatment of presumed intraocular tuberculosis in Japan. Ophthalmology 2002; 109: 851–857.
Basu S, Das T . Pitfalls in the management of TB-associated uveitis. Eye 2010; 24: 1681–1684.
Cheung CM, Chee SP . Jarisch-Herxheimer reaction: paradoxical worsening of tuberculosis chorioretinitis following initiation of anti-tuberculous therapy. Eye 2009; 23: 1472–1473.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Manousaridis, K., Ong, E., Stenton, C. et al. Clinical presentation, treatment, and outcomes in presumed intraocular tuberculosis: experience from Newcastle upon Tyne, UK. Eye 27, 480–486 (2013). https://doi.org/10.1038/eye.2013.11
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.11
Keywords
This article is cited by
-
Presumed ocular tuberculosis in the United Kingdom: a British Ophthalmological Surveillance Unit (BOSU) study
Eye (2020)
-
Presumed tuberculous uveitis in a university-based tertiary referral center in Saudi Arabia
International Ophthalmology (2019)
-
Tuberculosis and other causes of uveitis in Indonesia
Eye (2018)
-
Treatment outcome in patients with presumed tubercular uveitis at a tertiary referral eye care centre in Singapore
International Ophthalmology (2016)
-
Structural changes of the choroid in sarcoid- and tuberculosis-related granulomatous uveitis
Eye (2015)