Abstract
Purpose
To describe a new technique for performing maximum-depth anterior lamellar keratoplasty.
Methods
This was a case series study using a novel method. We introduce and describe a new sign (sunny-side up sign) that reveals the presence and extent of the air bubble at the Descemet membrane (DM)–stroma interface. We also report a novel technique to expand the bubble by injecting viscoelastic material into the bubble cavity and to excise the stromal tissues within the trephination area almost completely. The follow-up period ranged from 12 to 16 months. In all patients we recorded the best spectacle-corrected visual acuity, keratometry, and endothelial cell count preoperatively and postoperatively and the air bubble diameter using the sunny-side up sign.
Results
In eight of nine patients, a big bubble formed. The size of the air bubble ranged from 2 to 7 mm. All the bubbles were expanded to 8 mm and the bare DM throughout the trephination area was obtained in all cases. The postoperative mean keratometric readings were reduced compared with the preoperative mean keratometric readings. The BSCVA was increased postoperatively compared with the preoperative acuity. The difference between the preoperative and postoperative endothelial cell counts was not statistically significant.
Conclusions
The early outcomes in our series using the expanding bubble technique suggest that it is safe and easy in performing maximum-depth anterior lamellar keratoplasty.
Similar content being viewed by others
Introduction
During the last decade, renewed interest in lamellar keratoplasty has developed, after the procedure was abandoned in the 1960s. The introduction of techniques for deep lamellar dissection reduced the risk of irregularities and opacities at the interface compared with anterior lamellar dissection.1, 2 Many techniques have been introduced for accessing bare Descemet membrane (DM) as an ideal graft interface for achieving visual results comparable with penetrating keratoplasty.3, 4, 5, 6, 7, 8 However, the DM is not always bared successfully and its rupture sometimes mandates conversion to penetrating keratoplasty. One frequently used technique for exposing the DM is the big-bubble technique, which uses intrastromal air injection to create an air bubble between the DM and stroma.4 Excision of the overlying stromal tissues exposes the bare DM, but the diameter of the air bubble is usually smaller than the diameter of the trephination wound. Therefore, the stromal tissues that are located beyond the margins of the air bubble, but within the trephination margins, adhere to the underlying DM. This is a potential cause of DM rupture when the surgeon attempts to excise the peripheral stromal tissues. Conversely, leaving these stromal tissues without trying for excision may result in unwanted postoperative corneal astigmatism.
This study investigated a novel method of discriminating the margins of the air bubble and expanding the bubble to the desired size to separate the DM from the stromal tissues near the trephination wound and to excise the stromal remnants completely.
Materials and methods
Surgery was carried out on one eye in nine patients between August 2008 and January 2009. The patients were followed from 12 to 16 months (Table 1). In all cases, the underlying disorder was keratoconus. One surgeon (FD) performed all the surgeries. Manifest refraction (with spectacle correction), the best spectacle-corrected visual acuity (BSCVA) with a standard Snellen optotype, and keratometry using a Javal keratometer were measured at each visit. We considered mean keratometry as the average of two keratometric readings along the flattest and steepest meridians. Endothelial cell counts (ECCs) were recorded using a non-contact specular microscope (Topcon SP-2000p, Topcon, Tokyo, Japan) preoperatively and at 12 months postoperatively. Comparisons between preoperative and postoperative mean keratometry readings, ECCs, and BSCVA were performed using the Wilcoxon signed-rank test. Informed consent was obtained after we explained the possible consequences of the new technique. We certify that all applicable institutional regulations concerning the ethical use of human volunteers were followed during this research.
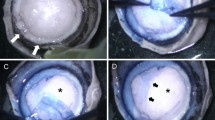
Partial-thickness trephination to a depth of 60–80% of the corneal thickness was performed using a calibrated trephine system (Katena Products, Denville, NJ, USA), as described by Anwar and Teichmann.4 A 27-gauge needle attached to an air-filled 5-ml syringe was used to inject air into the corneal stroma. The needle was inserted bevel down into the deep stroma in the paracentral cornea as in the big-bubble technique. After injecting the air, we performed paracentesis using a 15° stab knife (Eagle Laboratories, Rancho Cucamonga, CA, USA) in a semivertical direction as peripherally as possible to drain aqueous fluid. We used a crescent blade (Eagle Laboratories) to excise emphysematous stromal tissues in a lamellar keratectomy and reach the level of semi-clear stromal tissue. At this point, we widened the side port incision to 3 mm with a 15° stab knife and depressed the posterior lip of the side port incision to drain some aqueous fluid to make the anterior chamber obviously hypotensive. The widening of the incision was performed to make sure that there was not any air bubble in the anterior chamber resembling the big bubble at the DM–stroma interface. We used a dry Weck-Cel sponge (Beaver-Visitec International, Inc., Waltham, MA, USA) to depress the cornea in the paracentral area. When a big was bubble formed, the outline of the bubble was seen as a circular furrow around a dome-shaped elevation in the center, similar to the yolk of a sunny-side up egg (Figure 1). This sunny-side up sign helped us determine the presence and extent of the air bubble. At this point, we measured the horizontal diameter of the air bubble by assessing the distance between two opposing points on the encircling furrow on the horizontal meridian across the cornea. When no was big bubble formed, we continued by hydrating a localized area in the stroma with balanced salt solution and repeated air injection in the hydrated area using the same 27-gauge needle attached to an air-filled 5-ml syringe. This procedure had to be repeated several times in some patients before the sunny-side up sign could be detected (Figure 2). After the sunny-side up sign was seen, the 27-gauge needle that was used for air injection was attached to a syringe containing viscoelastic hydroxypropyl methylcellulose (Coatel; Bausch & Lomb, Madison, NJ, USA). Although we applied pressure to the plunger of the syringe and injected the viscoelastic material, we inserted the needle into the bubble cavity in the horizontal direction with a rapid movement. We continued to inject the viscoelastic material into the bubble cavity slowly and the ‘egg yolk’ expanded slowly (Figures 3a–c), until the demarcation furrow reached the trephination wound margins (Figure 3d). At this point, we stopped injecting viscoelastic material and withdrew the needle. We used the 15° stab knife to incise the roof of the bubble and excised the anterior wall of the bubble using curved blunt-tip corneal scissors (18010; Moria, Antony, France) around the trephination wound (Figure 4). After the DM was exposed to the full extent (Figures 5 and 6), we irrigated the exposed surface of DM with free flowing BSS solution for removing the viscoelastic material completely. The donor tissue was prepared by disinserting the DM and endothelium from peripheral insertion using a dry Weck-Cel sponge and wiping it off from the posterior surface of the corneal stroma. The donor cornea devoid of endothelium was cut to the desired size using a corneal punch (Katena Products). In all the nine patients, an 8-mm trephine was used for the recipient corneas and an 8.25-mm endothelial punch was used for the donor tissues. The donor button was secured in the recipient site with combined interrupted and continuous sutures (10-0 nylon).
(1) Big bubble is present between DM and posterior surface of the corneal stroma. (2) Viscoelastic material is injected into the bubble cavity and the bubble starts to expand. (3) Viscoelastic material is injected continuously within the bubble cavity and the bubble continues to expand. (4) The border of the expanding bubble reaches the trephination wound and the DM is detached completely within the trephination wound area. (5) The overlying corneal stromal tissue is excised leaving the bare DM surface throughout the trephination wound area.
In one patient, no big bubble formed after several attempts at air injection. In this case, we performed deep lamellar dissection with a crescent knife to the pre-Descemet level. We recognized a tiny bubble, measuring about 2 × 2 mm, at the DM–stroma interface. Under high microscopic magnification, we injected the viscoelastic material after inserting the tip of a 27-gauge needle attached to the viscoelastic syringe into the bubble; we then succeeded in creating an expanding bubble and continued as described above. Topical chloramphenicole drops and betamethasone drops were started at the next day following surgery four times a day. We followed the patients closely until complete epithelial healing (usually 5 days). In case of delayed epithelialization of the cornea, we used bandage soft lenses to promote epithelial healing. Topical preservative free lubricants were used as necessary. Topical antibiotics were discontinued after complete epithelialization of the cornea and topical steroids were tapered gradually and discontinued at the third postoperative month. Follow-up visits were at 2 weeks after surgery and then at monthly intervals for the next 4 months. Follow-up visits beyond that time were every 2 months until 1 year after surgery. We did not face any loose suture requiring replacement in the 4 postoperative months in our patients. After 4 months postoperatively, we removed corneal sutures selectively at the follow-up visits with the guide of computerized corneal topography and retinoscopy.
Results
The mean age of the nine patients was 23.55 years (range: 19–27 years); 55.5% of the patients were men. Of the treated eyes 66.6% were right and 33.4% were left. The corneal disorder was keratoconus in all patients. Bare DM was exposed to the full extent within the trephination wound margins in all cases. The diameter of the big bubble varied in our cases. We recorded the horizontal diameter of the air bubble after evacuating the aqueous fluid from the anterior chamber and revealing the sunny-side up appearance of the bubble. The diameters of the air bubbles ranged from 2 to 7 mm (mean, 5.78 mm; Table 1). The follow-up period ranged from 12 to 16 months. The corneal sutures had been removed in all patients until the last follow-up visit. We did not observe any retained viscoelastic material in our cases. Preoperatively, the BSCVA ranged from 1.3 to 0.5 log MAR (median, 1 log MAR). The BSCVA at the last follow-up visit ranged from 0.5 to 0.1 log MAR (median, 0.3 log MAR). The increase of the postoperative BSCVA compared with the preoperative BSCVA was statistically significant (P=0.0010. The preoperative mean keratometric readings ranged from 48.7 to 60.1 diopters (mean, 55.1 diopters) and the postoperative readings ranged from 42.7 to 45.9 diopters (mean, 45.74 diopters); this decrease was statistically significant (P=0.008). In addition, we found that eyes with various preoperative mean keratometric readings changed to eyes with similar postoperative mean keratometric measurements. The preoperative and postoperative ECCs ranged from 2620 to 3100 cells (mean, 2830 cells) and 2320 to 2990 cells (mean, 2733 cells), respectively; these differences were not statistically significant (P=0.53). However any dimpling of the cornea into the anterior chamber may cause some endothelial cell damage and it is prudent to protect endothelial cells by all possible means.
Discussion
Using surgical instruments such as blunt dissectors, various cannulas, and spatulas adjacent to the DM while trying to bare the membrane increases the risk of rupture. Surgeons performing corneal procedures frequently use the big-bubble technique to bare the DM. Although baring the DM in the central cornea is not difficult when the air bubble has been formed, the excision of the stromal tissues around the trephination margins is challenging and a risk of DM perforation exists. In this report, we present a technique for determining the presence and extent of the air bubble, manipulating the bubble to expand it to the desired size, and separating the DM from stromal tissue, fully exposing the DM in the trephination area. No perforation occurred in our patients and we succeeded in accessing a full extent of bare DM, which served as a bed for maximum-depth anterior lamellar keratoplasty.
Manche et al.7 reported a technique for performing deep lamellar dissection of the cornea using viscoelastic material. Melles et al.8 described the ‘air to endothelium light reflex’ as a guide for the plane of dissection after filling the anterior chamber with air. In 2000, Melles et al.9 presented the viscodissection technique. They injected viscoelastic material over the DM using the same ‘air to endothelium light reflex’ as a guide and induced DM detachment by the direct force of the injection. Senoo et al.10 introduced a technique to bare the DM by hydrodelamination through a corneoscleral flap and injecting viscoelastic material over a partially exposed DM to detach the membrane extensively against the air-filled anterior chamber. We presume that the presence of a large air bubble in the anterior chamber has a role in increasing the rate of DM perforation during viscodissection. A possible reason is that it generates counter pressure against the DM and resists DM detachment; when the detachment begins, it induces uneven pressure over the DM compared with a hypotensive fluid-filled anterior chamber. Yao11 reported a series of patients who underwent deep lamellar keratoplasty by injecting viscoelastic material over the bare DM. He used special forceps and a golf club-shaped diamond knife for hooking the stromal fibers at the trephination wound to expose the DM. Fournié et al.12 introduced a technique that forms an air bubble at the DM–stroma interface by making a corneal stromal nick after lamellar dissection and injecting air via a blunt cannula through the stromal nick. They refilled the air-filled cavity with viscoelastic material, they benefitted from the advantages of an extraocular procedure. Daneshgar et al.13 introduced the large-bubble technique, which facilitates excision of the stromal tissue completely within the trephination area.
Some investigators have reported using intracameral small air bubbles as a guide for detecting the presence of the big bubble.14, 15 To the best of our knowledge, no previous published study has mentioned the diameter of the air bubble in the DM–stroma interface during lamellar keratoplasty using the big-bubble technique. The sunny-side up sign described in this study could be a useful guide for detecting the big bubble and its extent in the DM–stroma interface.
Expanding the bubble technique provides direct visual control of the expansion of the detachment area to the desired size; therefore, excising the peripheral stromal tissues around the trephination margin could be less challenging. The participants in our study had full exposure of the DM in the trephination area.
In summary, many techniques have been introduced by surgeons performing lamellar corneal grafting to provide the best possible visual results for patients suffering from various corneal problems. We found the expanding bubble technique to be a safe and easy method to achieve this goal.

References
Arenas AE . Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea 1984/1985; 3: 21–28.
Malbran E . Lamellar keratoplasty in keratoconus. Int Ophthalmol Clin 1966; 6: 99–109.
Melles GRJ, Remeijer L, Geerards AJM, Beekhuis WH . The future of lamellar keratoplasty. Curr Opin Ophthalmol 1999; 10: 253–259.
Anwar M, Teichmann KD . Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg 2002; 28: 398–403.
Sugita J, Kondo J . Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol 1997; 81: 184–188.
Tan DT, Mehta JS . Future directions in lamellar corneal transplantation. Cornea 2007; 26 (suppl 1): S21–S28.
Manche EE, Holland GN, Maloney RK . Deep lamellar keratoplasty using viscoelastic dissection. Arch Ophthalmol 1999; 117: 1561–1565.
Melles GR, Lander F, Rietveld FJ, Remeijer L, Beekhuis WH, Binder PS . A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol 1999; 83: 327–333.
Melles GRJ, Remeijer L, Geerards AJM, Beekhuis WH . A quick surgical technique for deep, anterior lamellar keratoplasty using visco-dissection. Cornea 2000; 19: 427–432.
Senoo T, Chiba K, Terada O, Mori J, Kusama M, Hasegawa K et al. Deep lamellar keratoplasty by deep parenchyma detachment from the corneal limbs. Br J Ophthalmol 2005; 89: 1597–1600.
Yao YF . A novel technique for performing full-bed deep lamellar keratoplasty. Cornea 2008; 27: 19–24.
Fournié P, Malecaze F, Coullet J, Arné JL . Variant of the big-bubble technique in deep anterior lamellar keratoplasty. J Cataract Refract Surg 2007; 33: 371–375.
Behrooz MJ, Daneshgar F . ‘Large–bubble’ modification of the ‘big–bubble’ technique for performing maximum depth anterior lamellar keratoplasty. Cornea 2010; 29: 820–824.
Fontana L, Parente G, Tassinari G . Simple test to confirm cleavage with air between Descemet's membrane and stroma during big-bubble deep anterior lamellar keratoplasty. J Cataract Refract Surg 2007; 33: 570–572.
Shimazaki J . Double-bubble technique to facilitate Descemet membrane exposure in deep anterior lamellar keratoplasty. J Cataract Refract Surg 2010; 36: 193–196.
Acknowledgements
We thank Payman Daneshgar for his technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Daneshgar, F., Fallahtafti, M. ‘Expanding bubble’ modification of ‘big-bubble’ technique for performing maximum-depth anterior lamellar keratoplasty. Eye 25, 803–808 (2011). https://doi.org/10.1038/eye.2011.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.72