Abstract
Purpose
To study the incidence, clinical findings, and tumour characteristics of posterior uveal melanoma in Western Norway, and to report the results of a consistent treatment strategy (I-125 brachytherapy or primary enucleation) over a 13-year period.
Methods
The clinical records of all patients with posterior uveal melanoma referred between January 1993 and December 2005 were reviewed. Clinical data, radiation parameters, visual outcome, and mortality were analysed in a dedicated database.
Results
The study included 111 consecutive patients. The annual age-adjusted incidence (per million population) of posterior uveal melanoma was 8.5 for women and 8.9 for men. Fifty-six patients underwent I-125 brachytherapy, 52 were enucleated, and three received no treatment. The median follow-up time was 36 months (mean, 52 months; range, 2 months to 13 years). In the brachytherapy group, two eyes were enucleated owing to tumour recurrence and two because of neovascular glaucoma. A visual acuity of 0.1 or better, present in 87% of the patients before brachytherapy, was retained in 40% after a median follow-up of 61 months. After brachytherapy, the 5- and 10-year melanoma-specific mortality rates were 13.4 and 23.8%, respectively. The corresponding mortality rates for patients treated with primary enucleation were 49.5 and 49.5%.
Conclusion
After brachytherapy, many patients lost useful vision due to radiation-induced complications. The probability of retaining the eye was high and only two patients experienced recurrent tumour growth. The mortality rates compare well with published series, and the differences in tumour size explain the difference in mortality between the two treatment groups.
Similar content being viewed by others
Introduction
Various treatments for posterior uveal melanoma, including brachytherapy with different radioactive sources, charged particle radiation, and local resection, have been introduced over the past decades. Despite this, there has been no general improvement in the survival rates.1 However, the Collaborative Ocular Melanoma Study has shown that there is no significant difference in melanoma-related mortality when comparing the eye-sparing treatment of episcleral brachytherapy with primary enucleation,2 which are still the two most commonly employed methods of treating posterior uveal melanoma. Episcleral brachytherapy is the preferred method for small- and medium-sized tumours, whereas enucleation is recommended for large tumours and tumours surrounding the optic nerve.3 The aims of this study were to analyse the incidence, clinical findings, and tumour characteristics of patients in Western Norway, and to review our results of posterior uveal melanomas treated with a consistent strategy over a period of 13 years.
Materials and methods
The clinical records of all patients with posterior uveal melanoma referred to Haukeland University Hospital between 1 January 1993 and 31 December 2005 were reviewed. Haukeland University Hospital is the regional hospital for the three western counties of Norway (Rogaland, Hordaland, and Sogn og Fjordane). To analyse the incidence of posterior uveal melanoma in Western Norway, the number of residents who were treated elsewhere was collected from the Cancer Registry of Norway and by reviewing the clinical records at other hospitals in the region. Population figures by age, gender, county and calendar period were obtained from Statistics Norway. The study was approved by The Norwegian Social Science Data Services, and followed the official ethical regulations for clinical research.
Preoperative data included age, gender, best-corrected Snellen visual acuity, tumour location, distance from the posterior tumour border to the foveola and the edge of the optic disc, and largest basal tumour diameter and height. The tumour size was scored according to both the TNM classification4 and the COMS criteria.5 The presence of exudative retinal detachment was defined as subretinal fluid involving at least one quadrant of the fundus. Bruch's membrane was considered ruptured if ultrasonography revealed a mushroom-shaped tumour.
Our preferred treatment strategy was to perform iodine-125 brachytherapy for tumours measuring <10 mm in height and primary enucleation for larger tumours and tumours surrounding more than one-quarter of the optic disc circumference. However, the choice of treatment was to a great extent dependent on tumour location, the possibility of reducing radiation damage to the optic nerve and retina by plaque design and seed positioning, the status of the fellow eye, and the patient's preference. The data from all tumours eligible for brachytherapy were analysed in a three-dimensional treatment planning program (Plaque Simulator, BEBIG Isotopen-und Medizintechnik GmbH, Berlin, Germany) for accurate tumour location and dosimetry.6, 7 The prescription point was tumour height plus 1 mm for the thickness of sclera, and the total radiation dose to the tumour apex was usually set to 100 Gy including a safety margin of 2 mm around the tumour base. To minimize the dose to the optic disc and macula, some tumours were treated with no or minimal safety margin towards these sensitive structures, and transscleral illumination was used during surgery for accurate plaque placement.8
Radiation data included hours of radiation exposure, and radiation dose (Gy) and dose rate (cGy/h) to various ocular structures. Follow-up data comprised best-corrected visual acuity, tumour radiation response and occurrence of ocular complications or tumour regrowth. Radiation-induced complications were defined according to Gündüz et al,9 and tumour recurrence was defined as any documented tumour growth. For patients treated with enucleation, histopathologic examination determined the classification of tumour cells grouped into spindle, mixed, or epithelioid cell types,10 scleral invasion of melanoma cells, and the integrity of Bruch's membrane. Finally, the dates of the last visit and the dates and causes of death were recorded. The cause of death was determined by analysing both the hospital files and the general practitioner clinical records. The end of follow-up was 31 December 2005.
The Kaplan–Meier method was used to analyse the eye retention rate, all-cause mortality rates and melanoma-specific mortality rates. Prognostic factors for radiation maculopathy and mortality were estimated by Cox regression analyses. The difference in incidence of posterior uveal melanoma between genders was analysed by the χ2 test. Age-adjusted incidence rates were calculated by a direct method using weights according to the European Standard Population. The statistical analyses were made by the software package SPSS, version 13.0 (SPSS Inc., Chicago, IL, USA). P-values <0.05 were considered to be statistically significant.
Results
The study included 111 consecutive patients (60 women and 51 men) with posterior uveal melanoma. Western Norway was the area of residence for 102 patients, and nine patients were referred from other parts of the country. The median age at time of diagnosis was 66 years (range, 24–96 years). The main tumour site was the choroid in 100 patients (90%) and the ciliary body in 11 patients (10%). Fifty-six patients (50%) were treated with brachytherapy, and 52 patients (47%) underwent primary enucleation (Table 1). Three patients (3%) received no specific ocular treatment (two had melanoma metastases at the time of diagnosis, and one refused any kind of treatment). The median follow-up time was 36 months (mean, 52 months; range, 2 months to 13 years).
At the end of follow-up, the vital status was known for all the 111 patients, and a total of 42 patients (38%) were dead. Twenty-eight deaths (25%) were melanoma related, 27 of these due to systemic metastases and one because of inoperable melanoma recurrence in the orbit with tumour growth into the skull base and cavernous sinus. The 5- and 10-year melanoma-specific mortality rates were 30.4% (95% confidence interval (CI), 20.2–40.6) and 36.4% (95% CI, 23.9–48.9), respectively. The corresponding all-cause mortality rates were 41.6% (95% CI, 31.2–52.0) and 49.7% (95% CI, 37.7–61.7). These figures include the two patients with metastases at the time of diagnosis, and the one who refused treatment. None of the surviving patients had known metastases at the end of follow-up. By multivariate Cox regression analysis, large basal tumour diameter emerged as the only significant predictive factor for the development of melanoma metastases (P<0.001).
Brachytherapy group
Postoperatively, there was a gradual decrease of visual acuity with the strongest decrease during the first 3 years after brachytherapy (Figure 1). Before brachytherapy, visual acuity (excluding four patients that subsequently required enucleation) was 6/4 to 6/12 in 31 patients (60%), 6/18 to 6/60 in 14 (27%), and worse than 6/60 in seven (13%). At the latest visit after brachytherapy, the distribution was 6/4 to 6/12 in 10 patients (19%), 6/18 to 6/60 in 11 (21%), and worse than 6/60 in 31 (60%). Twenty-three patients (44%) preserved a visual acuity within two Snellen lines of preoperative levels. The median time interval from treatment to the last evaluation of visual acuity was 61 months (range, 3 months to 12 years).
The radiation dose and dose rate to various ocular structures are shown in Table 2. The median radiation time was 117 h (range, 61–214 h). One patient with a tumour height of 12 mm insisted on brachytherapy, which necessitated the extraordinary high doses of 1449 Gy to the tumour base and 2340 Gy to the scleral surface. Some patients had more than one radiation-related complication, and the most frequent complication was cataract in 17 patients (30%), followed by maculopathy in 16 (29%), optic neuropathy in nine (16%), neovascular glaucoma in eight (14%), and vitreous haemorrhage in four (7%). By univariate Cox regression analysis, the only significant predictive factors for radiation maculopathy were increased tumour height (P=0.02) and basal diameter (P=0.04).
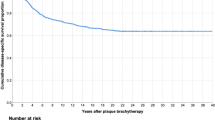
A total of four eyes (7%) were enucleated after brachytherapy. Two of these eyes were enucleated because of tumour recurrence 14 and 15 months after brachytherapy, and two eyes were enucleated owing to uncontrollable neovascular glaucoma. The 5- and 10-year eye retention rates after brachytherapy were 90.6% (95% CI, 81.8–99.4) (Figure 2).
Fourteen patients (25%) were dead at the end of follow-up. Eight patients (14%) died of melanoma metastases. The 5- and 10-year melanoma-specific mortality rates for the patients undergoing brachytherapy were 13.4% (95% CI, 3.4–23.4) and 23.8% (95% CI, 6.9–40.7), respectively (Figure 3a). The corresponding all-cause mortality rates were 21.2% (95% CI, 9.4–33.0) and 36.0% (95% CI, 19.0–53.1).
Enucleation group
Before enucleation, visual acuity was 6/4 to 6/12 in 11 patients (21%), 6/18 to 6/60 in 17 (33%), and worse than 6/60 in 24 (46%). Histopathologic examination showed that 31 tumours (59%) were of spindle cell type, 17 (33%) of mixed cell type, and four (8%) were pure epithelioid cell tumours. Scleral invasion of tumour cells was found in 23 (44%) of the enucleated eyes, with extrascleral extension in four eyes (8%). Rupture of Bruch's membrane was observed in 19 eyes (37%).
Twenty-five patients (48%) were dead at the end of follow-up, and 18 deaths (35%) were melanoma-related. The 5- and 10-year melanoma-specific mortality rates for the patients treated with primary enucleation were 49.5% (95% CI, 31.9–67.1) and 49.5% (95% CI, 31.9–67.1), respectively (Figure 3b). The corresponding all-cause mortality rates were 63.1% (95% CI, 47.2–79.0) and 63.1% (95% CI, 47.2–79.0).
Incidence
During the study period, 115 patients living in the three counties of Western Norway were diagnosed with posterior uveal melanoma. One hundred and two of these patients were treated at Haukeland University Hospital and 13 were treated at other hospitals in Norway. The crude incidence rate (per million population per year) was 10.5 for women and 9.0 for men. The corresponding age-adjusted incidence rates were 8.5 and 8.9. The age-specific incidence was higher in men (23.2) than in women (16.7) at 50–69 years of age, but shifted towards a higher incidence in women (43.5) compared with the incidence in men (39.2) in the age group 70–89 years. There was no statistically significant difference in the incidence between genders in any age group.
Discussion
In this study, we present a consecutive series of patients with posterior uveal melanoma treated with episcleral brachytherapy or primary enucleation. As the choice between I-125 brachytherapy and enucleation was based on clinical criteria and patient preference, a direct comparison of the treatment results between the two groups is inappropriate. However, the study reflects what can be achieved with current methods of treatment in an unselected group of patients with a lengthy and complete follow-up.
In the brachytherapy group, a relatively large proportion of the patients lost useful vision during follow-up. In 16% of the patients the tumour was classified as large and 20% had a tumour that measured more than 8 mm in thickness, which both are factors associated with ocular complications and a poor visual outcome in most patients.11, 12 The visual results in our study may also be influenced by the prescription dose of 100 Gy to the tumour apex, which is somewhat higher than that recently recommended.13 Radiation-related maculopathy and optic neuropathy were the main causes of visual loss. The patients who developed neuropathy had a median dose of 55 Gy to the centre of the optic disc. According to Lommatzsch et al,14 radiation doses above 50 Gy are significantly correlated to optic neuropathy. Surprisingly, we found that the patients with radiation maculopathy had a median dose to the fovea of 49 Gy, which was only slightly higher than the median foveal dose of 45 Gy for the whole group. Gragoudas et al15 have shown that any radiation exposure of the macula increases the risk of maculopathy, with the risk rising steadily to and levelling off after approximately 40 Gy. This probably reflects that the macula is a sensitive structure, not only to irradiation but also to other factors such as inflammation, exudation, and vascular changes in an adjacent tumour after brachytherapy. The regression of a large tumour may damage the macula regardless of radiation dose, or it can make the macula more vulnerable to the radiation already given during brachytherapy. In this study, large tumour diameter and height were the only significant risk factors for radiation maculopathy in the univariate analyses.
The local tumour recurrence rate of 3.6% compares favourably with other reports.16, 17, 18, 19 We believe that the consistent use of iodine as the radioactive source and the relatively high tumour apex dose of 100 Gy have contributed to the low recurrence rate. As tumour regrowth has been associated with a poor survival prognosis,9, 20, 21 an effective local tumour control is essential in brachytherapy of uveal melanoma. When differences in patient selection, tumour characteristics, and follow-up are taken into consideration, the mortality rates in our study are generally comparable to those reported by others.2, 9, 18, 22, 23 Several studies have shown that the size of the uveal melanoma is a risk factor for subsequent metastases.22, 24, 25 In the multivariate analyses, we found that large basal tumour diameter was the only significant predictive factor for metastatic disease. The differences in tumour size could therefore explain the difference between the mortality curves in the two treatment groups.
The incidence of uveal melanoma in the Scandinavian population is generally considered to be high.26, 27 Contrary to previous reports, which have shown a slight predominance of males,28 we found no statistically significant differences in the crude or age-adjusted incidence rates between genders. Although not fully comparable, the annual incidence of ocular melanoma in Norway has been reported to be 7.0 for women and 9.0 for men,29 which indicates that the incidence of uveal melanoma has been relatively stable over the past several decades.
In summary, we have documented our experience with brachytherapy and primary enucleation in the treatment of posterior uveal melanoma. The mortality rates compare well with previously published series. The probability of retaining the eye after brachytherapy was high, and only two patients experienced recurrent tumour growth. On the basis of these results, we maintain a preference for I-125 brachytherapy with three-dimensional treatment planning, and hope to improve the visual outcome without compromising tumour control by reducing the planned radiation dose.
References
Singh AD, Topham A . Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology 2003; 110: 962–965.
Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol 2001; 119: 969–982.
Shields JA, Shields CL . Atlas of intraocular tumors. Lippincott Williams & Wilkins: Philadelphia, 1999.
UICC, International Union against Cancer. TNM Atlas. Illustrated guide to the TNM/pTNM classification of malignant tumours, 3rd ed., 2nd revision. Springer-Verlag: Berlin, 1992.
Collaborative Ocular Melanoma Study Group. Comparison of clinical, echographic, and histopathological measurements from eyes with medium-sized choroidal melanoma in the collaborative ocular melanoma study. COMS Report No.21. Arch Ophthalmol 2003; 121: 1163–1171.
Astrahan MA, Luxton G, Pu Q, Petrovich Z . Conformal episcleral plaque therapy. Int J Radiat Oncol Biol Phys 1997; 39: 505–519.
Knutsen S, Hafslund R, Monge OR, Valen H, Muren LP, Rekstad BL et al. Dosimetric verification of a dedicated 3D treatment planning system for episcleral plaque therapy. Int J Radiat Oncol Biol Phys 2001; 51: 1159–1166.
Krohn J, Seland JH, Monge OR, Rekstad BL . Transillumination for accurate placement of radioactive plaques in brachytherapy of choroidal melanoma. Am J Ophthalmol 2001; 132: 418–419.
Gündüz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW . Radiation complications and tumor control after plaque radiotherapy of choroidal melanoma with macular involvement. Am J Ophthalmol 1999; 127: 579–589.
Callender GR . Malignant melanotic tumors of the eye: a study of histologic types in 111 cases. Trans Am Acad Ophthalmol Otolaryngol 1931; 36: 131–142.
Puusaari I, Heikkonen J, Kivelä T . Ocular complications after iodine brachytherapy for large uveal melanomas. Ophthalmology 2004; 111: 1768–1777.
Shields CL, Naseripour M, Cater J, Shields JA, Demirci H, Youseff A et al. Plaque radiotherapy for large posterior uveal melanomas (⩾8-mm thick) in 354 consecutive patients. Ophthalmology 2002; 109: 1838–1849.
Nag S, Quivey JM, Earle JD, Followill D, Fontanesi J, Finger PT . The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys 2003; 56: 544–555.
Lommatzsch PK, Alberti W, Lommatzsch R, Rohrwacher F . Radiation effects on the optic nerve observed after brachytherapy of choroidal melanomas with 106Ru/106Rh plaques. Graefes Arch Clin Exp Ophthalmol 1994; 232: 482–487.
Gragoudas ES, Li W, Lane AM, Munzenrider J, Egan KM . Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology 1999; 106: 1571–1577.
Shields CL, Shields JA, Karlsson U, Markoe AM, Brady LW . Reasons for enucleation after plaque radiotherapy for posterior uveal melanoma. Ophthalmology 1989; 96: 919–924.
Char DH, Quivey JM, Castro JR, Kroll S, Phillips T . Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology 1993; 100: 1547–1554.
Packer S, Stoller S, Lesser ML, Mandel FS, Finger PT . Long-term results of iodine 125 irradiation of uveal melanoma. Ophthalmology 1992; 99: 767–773.
Wilson MW, Hungerford JL . Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology 1999; 106: 1579–1587.
Karlsson UL, Augsburger JJ, Shields JA, Markoe AM, Brady LW, Woodleigh R . Recurrence of posterior uveal melanoma after 60Co episcleral plaque therapy. Ophthalmology 1989; 96: 382–388.
Vrabec TR, Augsburger JJ, Gamel JW, Brady LW, Hernandez C, Woodleigh R . Impact of local tumor relapse on patient survival after cobalt 60 plaque radiotherapy. Ophthalmology 1991; 98: 984–988.
Seregard S, Kock E . Prognostic indicators following enucleation for posterior uveal melanoma. A multivariate analysis of long-term survival with minimized loss to follow-up. Acta Ophthalmol Scand 1995; 73: 340–344.
Cohen VML, Carter MJ, Kemeny A, Radatz M, Rennie IG . Metastasis-free survival following treatment for uveal melanoma with either stereotactic radiosurgery or enucleation. Acta Ophthalmol Scand 2003; 81: 383–388.
Isager P, Ehlers N, Overgaard J . Prognostic factors for survival after enucleation for choroidal and ciliary body melanomas. Acta Ophthalmol Scand 2004; 82: 517–525.
Shammas HF, Blodi FC . Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol 1977; 95: 63–69.
Bergman L, Seregard S, Nilsson B, Ringborg U, Lundell G, Ragnarsson-Olding B . Incidence of uveal melanoma in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci 2002; 43: 2579–2583.
Isager P, Østerlind A, Engholm G, Heegaard S, Lindegaard J, Overgaard J et al. Uveal and conjunctival malignant melanoma in Denmark, 1943–97: Incidence and validation study. Ophthalmic Epidemiol 2005; 12: 223–232.
Singh AD, Topham A . Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology 2003; 110: 956–961.
Mork T . Malignant neoplasms of the eye in Norway. Incidence, treatment and prognosis. Acta Ophthalmol (Copenh) 1961; 39: 824–831.
Acknowledgements
The study was supported by grants from The Norwegian Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krohn, J., Monge, O., Skorpen, T. et al. Posterior uveal melanoma treated with I-125 brachytherapy or primary enucleation. Eye 22, 1398–1403 (2008). https://doi.org/10.1038/sj.eye.6702911
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702911
Keywords
This article is cited by
-
Topography and clinical features of iris melanoma
BMC Ophthalmology (2022)
-
Ocular complications following I-125 brachytherapy for choroidal melanoma
Eye (2009)
-
Initial results of fractionated CyberKnife radiosurgery for uveal melanoma
Journal of Neuro-Oncology (2009)