Abstract
Correct diagnosis and successful therapy are extremely important to enjoy a healthy life when suffering from a disease. To achieve these aims, various cutting-edge technologies have been designed and fabricated to diagnose and treat specific diseases. Among these technologies, aptamer–nanomaterial hybrids have received considerable attention from scientists and doctors because they have numerous advantages over other methods, such as good biocompatibility, low immunogenicity and controllable selectivity. In particular, aptamers, oligonucleic acids or peptides that bind to a specific target molecule, are regarded as outstanding biomolecules. In this review, several screening techniques for aptamers, also called systematic evolution of ligands by exponential enrichment (SELEX) methods, are introduced, and diagnostic and therapeutic aptamer applications are also presented. Furthermore, we describe diverse aptamer–nanomaterial conjugate designs and their applications for diagnosis and therapy.
Similar content being viewed by others
Introduction
For several decades, nanotechnology has made remarkable advancements in various fields, such as electricity, communication, material science, manufacturing, medical treatment and life sciences. In particular, the convergence of biomolecules and nanomaterials has led to a breakthrough in the diagnosis and therapy of specific diseases, including cancers. Biocompatible molecules, like DNA, RNA and peptides, enable specific targeting and imaging. Moreover, the unique spectroscopic and thermotherapeutic properties of nanomaterials provide superior advantages for sensing, imaging and therapeutic applications. As shown in Figure 1, biomolecule–nanomaterial hybrids are readily applicable to many areas.
In particular, accurate diagnosis and effective therapy have been regarded as key factors in the medical field. The development of next-generation materials for successful diagnosis and therapy has been an important research focus. Among many substances, aptamers are recognized as one of several remarkable agents because of their valuable properties.1, 2 Aptamers are oligonucleic acids or peptides that have high sensitivity and robust selectivity toward several types of target molecules, including proteins, nucleotides, peptides, antibiotics, small molecules and cells.3 Small nucleic acid aptamers exhibit good stability in severe conditions, and peptide aptamers have suitable structures that interact with target molecules. All types of aptamers contain a variable loop and stem region that bind to a specific pocket of target molecules. Aptamers have diverse advantages over antibodies, including small size, easy modification and high stability in harsh physical and chemical environments, as well as rapid and economical production, no batch-to-batch variation, low immunogenicity and high flexibility.4 Therefore, aptamers are regarded as excellent substitutes for antibodies during targeting and imaging in medical sciences, and are used in an assortment of fields.
The development of numerous nanomaterials has accelerated advancements in diagnosis and therapy.5 A variety of nanomaterials, such as hydrogels, metallic nanoparticles, silica nanoparticles and carbon materials, have ideal characteristics, including controllable physical and chemical properties, a large surface area, robust biocompatibility and outstanding stability.6, 7 Even though nanomaterials themselves can be used as diagnostic and therapeutic agents, they lack selective targeting ability. Therefore, a number of aptamer–nanomaterial complexes have been designed and applied to multiple areas.8, 9, 10
In this review, we focus on an overview of recent improvements for aptamer and aptamer–nanomaterial conjugates, as well as their applications for the diagnosis and treatment of several diseases.
In vitro screening for aptamers
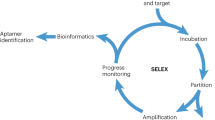
Target-specific aptamers are selected in vitro using a process, called systematic evolution of ligands by exponential enrichment (SELEX).11, 12 As represented in Figure 2a, SELEX is a repetition of four main steps: target incubation with aptamer libraries encoding random sequences, usually 30–50 mers, and the primer-binding site; elution of bound libraries; amplification with polymerase chain reaction; and single-stranded oligonucleotide separation. This procedure is generally repeated 10–15 times, and then the selected aptamer candidates are analyzed using cloning techniques.
The SELEX procedure. (a) A schematic illustration for general SELEX. It consists of four steps: incubation of target molecules with libraries, elution of bound libraries, amplification of the libraries and separation of the libraries for the next round. (b) A schematic illustration of cell-based SELEX. Negative selection is required for each round.
To develop aptamers to recognize proteins or small molecules, five types of SELEX have been generally applied. The first method, nitrocellulose membrane filtration-based SELEX, has been used since 1990, when it was designed by Tuerk and Gold.12 The role of nitrocellulose membrane is to only filter libraries that can bind to the protein based on specific protein-binding affinity. However, this method is limited to only proteins because the nitrocellulose membrane is capable of binding proteins. The second method is affinity chromatography-based SELEX, which uses the specific interaction between immobilized beads and their target molecules. When the library is loaded onto the target-immobilized affinity column, the non-binding fraction is washed via flow-through, and a binding library is obtained.13 However, this method is confined because it requires a functional group or affinity tag on the target molecule. In addition, functionalized magnetic bead-based SELEX has frequently been applied to discover new aptamer candidates. Target molecules are immobilized on a magnetic bead using a covalent bond, a specific interaction or an electrostatic interaction between a substrate on the beads and a specific tag on the target molecule.14 Easy and rapid separation of the library bound to the target using an external magnet makes this SELEX method valuable. In addition, capillary electrophoresis-based SELEX has many advantages, such as fast separation, high resolution, large capacity and a small amount of samples.15 Under an electric field, each molecule is separated based on its charge, hydrodynamic radius and frictional force. SELEX can be accomplished within a few cycles using this method. Finally, SELEX is performed with a microfluidic chip-based technique, which offers good selection efficiency on a small scale and rapid separation via automatic continuous-flow.16 The development of microfluidic chip technology has the potential to enable fast and convenient SELEX.
On the other hand, aptamers for comparatively large targets, such as cancer cells, have been identified via another method as described in Figure 2b. Because almost all aptamers recognize the surface biomarkers on cancers, this method is termed ‘cell-based SELEX’.17 Miscellaneous hindrances on the cell surface, such as many non-target proteins, glycosides and various receptors, make negative selection essential during each cycle. Although this method requires multiple cycles, it is significant enough to generate aptamers identifying target cells that do not overexpress biomarkers.
In recent years, the convergence of new technologies and SELEX has allowed effective and economic aptamer screening. Computational approaches have been introduced to SELEX for the prediction and optimization of aptamer structures, binding affinity and thermodynamic characteristics.18 Several programs, such as Resetta,19 FR3D20 and MPBind,21 have been developed and utilized for 3D structure predictions of aptamers.22 In addition, a high-throughput sequencing technique has been used for its high efficiency and extensive applicability. High-throughput sequencing offers the complete sequence analysis of aptamer candidates, exact quantification, the comparison of binding motifs among each sequence and the characterization of binding affinities.23, 24 Such substantial advances in SELEX methods have generated noteworthy outgrowth in the design and identification of new aptamers, and further modifications of SELEX have been successful in the innovations for screening aptamers.
Diagnostic applications of aptamers
Highly sensitive and selective aptamers have boundless potential applications as diagnostic molecules. Diagnostic methods are commonly categorized according to three approaches based on detection principles. In the first approach, aptamers are often used to fabricate electrochemical biosensors, which can provide an accurate, rapid and sensitive diagnosis. Figure 3a depicts an example of electrochemical impedance spectroscopy using aptamers.25 These electrochemical detection methods, including cyclic voltammetry and differential pulse voltammetry, have many advantages, such as reproducibility, a rapid response, no probe labeling, high sensitivity and selectivity, and no need for high-cost instrumentation.26 In addition, aptamers are valuable probes in colorimetry. Detection in colorimetry uses color changes in some of the particles, and a gold nanoparticle (AuNP) is one of the best indicators for this method. Its unique characteristics, such as a positive charge, color change according to interparticle distance and absorption of the thiol group, enable rapid and convenient detection. As shown in Figure 3b, the target protein can simply be detected by the AuNP color change.27 The presence of the target protein is confirmed by the competitive binding of the polymers. Fluorescence-based diagnosis is also used in aptamer-based detection, which is a powerful diagnostic and bioanalytical platform. The introduction of aptamers to fluorometry has created a synergistic effect. Recently, Roncancio et al.28 reported a rapid and specific aptamer-based platform for one-step cocaine detection using minimal reagent requirements, and verified that this sensor detects cocaine with a detection limit of 200 nM, which is 50-fold lower than the existing limit (Figure 3c).
Various applications of aptamers for detection. (a) Electrochemical impedance spectroscopic detection for MUC1. This image was adapted from Chen et al.25 (b) Colorimetric detection. This image was adapted from Jeon et al.27 (c) Fluorescent detection. This image was adapted from Roncancio et al.28 Apt, aptamer; ATMND, 2-amino-5,6,7-trimethyl-1,8-naphthyridine; PDDA, poly(diallyldimethylammonium chloride); pLDH, Plasmodium lactate dehydrogenase; PoPD, poly(o-phenylenediamine); TEOS, tetraethyl orthosilicate; Thi, thionine.
Moreover, it is well-known that aptamers have been commonly utilized for other diagnostic research, such as single-wall nanotube-based detection, surface acoustic waves, surface plasmon resonance, micro cantilever sensing and quartz crystal microbalance.29, 30, 31, 32 Aptamers are novel and invaluable next-generation targeting probes that are readily applicable to target-specific diagnosis.
Therapeutic applications of aptamers
Some aptamers have shown high binding affinity for target molecules and an antagonistic effect on specific functions of the targets, allowing their use as probes and drugs. Dating from the discovery of Mucugen, a vascular endothelial growth factor-specific aptamer, in 2004 and its approval by the US Food and Drug Administration as a new drug for treating age-related macular degeneration, diverse aptamers have been recognized as effective drugs for specific diseases. For example, ARC1779 (Archemix, Cambridge, MA, USA) targets activated von Willebrand Factor and was designed as a drug for purpura (Figure 4a). NOX-A12 (NOXXON Pharma, Berlin, Germany), which targets CXCL12, was developed to treat multiple myeloma and non-Hodgkin’s lymphoma (Figure 4b).33, 34 Aptamers themselves can be valuable, useful and effective medicines.
In addition, aptamers have been used as good drug carriers. The intercalating properties of nucleic acid aptamers mean that they are selective and sensitive delivery vehicles. On the basis of the fact that doxorubicin, an anti-cancer drug, can easily intercalate into an A10 aptamer that recognizes prostate-specific membrane antigen (PSMA), which is overexpressed prostate cancer, a remarkable drug carrier was designed.35 The dual-aptamer complex is specific for both PSMA (+) and (−) prostate cancer cells and delivers doxorubicin to the target cells incredibly effectively and selectively. Furthermore, aptamers can be used only as targeting molecules. For instance, the combination of an aptamer and siRNA generates marvelous therapeutic effects.36 Direct conjugation between the targeting probe and siRNA provides a simple and convenient preparation method for the drug. Countless studies have been vigorously performed examining aptamers.
Aptamer–nanomaterial complexes in diagnosis
Quantum dots (QDs), semiconductor nanocrystals, have been gradually used in biological and medical diagnoses owing to their inimitable features, such as low photobleaching, high resistance to harsh chemical degradation, high-quantum yield and broad absorption with narrow photoluminescence spectra.37 In particular, QDs have been regarded as excellent Förster resonance energy transfer (FRET) donors. The basic principle in FRET-based sensors is a target-dependent change in fluorescence signal caused by the distance between a donor and an acceptor. Representative FRET-based sensors using QDs are highlighted in Figure 5a.38 Good FRET properties make aptamer-modified QDs particularly useful as probes in diagnosis. In 2009, Zhang et al.39 designed a cocaine-specific detection platform. The Cyanine5-modified cocaine aptamer is hybridized to its complementary DNA, which is immobilized on the surface of the QD, resulting in huge FRET fluorescence. The selective binding between cocaine and the cocaine aptamer triggers a decrease in Cyanine5 fluorescence. Therefore, QD-based detection offers highly sensitive and selective detection of target molecules.
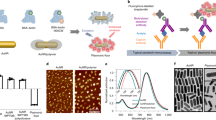
Diagnostic applications of biomolecule–nanomaterial complexes. (a) Three types of FRET-based detection using QDs: detection using quencher-modified oligonucleotides, detection using fluorophore-modified oligonucleotides and detection using intercalating dyes. This image was adapted from Zhou.38 (b) Detection platform for breast cancer using dual-aptamer-modified SiNPs. HER2(+) or MUC1(+) breast cancer cells are selectively separated using dual-aptamer-modified magnetic beads, and SiNP conjugates are incubated with the magnetic beads-cell complexes. Breast cancer cells are quantified based on fluorescence from dyes inside the SiNPs. This image was adapted from Jo et al.45 Ex, excitation.
AuNPs are one of the best sensing nanomaterials and stable metallic nanoparticles. They retain many superior advantages over other nanomaterials for various reasons, including their tunable size and shape, easy and economic preparation, size-dependent electronic and optical properties, good biocompatibility, high intracellular stability, quenching ability and strong interaction with thiol functional groups.40 Therefore, studies investigating AuNP-based detection have been performed for a long time. Aptamers can provide targeting abilities to AuNPs, therefore, aptamer-modified AuNPs are extremely useful diagnostic complexes. Target-specific AuNP probes have been designed to have various characteristics in countless assays, including colorimetry, fluorometry, electrochemical detection, surface plasmon resonance, surface-enhanced Raman spectroscopy and quartz crystal microbalance. For instance, Medley et al.41 designed a simple and rapid detection method for cancer cells using aptamer-modified AuNPs. Aptamer-modified AuNPs selectively bind to target cells, and the interparticle distance decreases between the AuNPs, which generates discernible color changes from red to violet, leading to the straightforward detection of cells. The superquenching capacity of AuNPs is also a unique feature. Song et al.42 developed an ampicillin-specific AuNP sensor. When 5’-fluorescein amidite-modified aptamers are adsorbed to the AuNP surface via columbic interaction, quenching of the fluorophore occurs. Competitive binding of the ampicillin aptamer to AuNPs and ampicillin enables the restoration of fluorescence from fluorescein amidite, resulting in the sensitive quantification of ampicillin levels. In addition to these, aptamer-AuNPs have been applied to diagnose several specific diseases and to identify several molecules as well.
It has been known that silica nanoparticles (SiNPs) possess facile fabrication, easy surface modification, good biocompatibility, economic synthesis, hydrophilic properties and sterically hinder nucleases, which cleave DNA on the surface of the particle.43 In particular, dye-doped SiNPs have received considerable attention as excellent probes due to their high-fluorescence intensity, robust signal and robust resistance to photobleaching, which hinders the fluorescence signals from almost all organic dyes. Coulombic interactions between positively charged dye molecules and the negatively charged silica matrix reinforces the resistance of SiNPs against photobleaching. Aptamer-modified, dye-doped SiNPs are actively utilized in many diagnosis studies. Mucin 1 (MUC1) aptamer-immobilized, dye-entrapped SiNPs were synthesized by Cai et al.44 and exhibited an incredibly steady fluorescence signal and high selectivity toward MUC1 (+) cell lines only. Recently, the diagnosis of breast cancer using dual-aptamer-functionalized SiNPs was successfully performed.45 As shown in Figure 5b, using dye-doped SiNPs modified with a MUC1 aptamer and a human epidermal growth factor receptor 2 aptamer, various types of breast cancer cell lines overexpressing MUC1 or human epidermal growth factor receptor 2 were quantified. Therefore, specific aptamer-modified SiNPs can function as beneficial diagnostic tools for certain targets.
In contrast with other nanoparticles, magnetic nanoparticles (MNPs) have a peculiar trait that improves the magnetic resonance of adjacent water.46 In particular, the aggregation of MNPs can cause the coupling of spin magnetic moments and produces strong local magnetic fields, resulting in accelerated dephasing of water protons.47 A change in proton relaxation times, produced by a decrease of transverse or spin–spin relaxation times (T2), enables the detection of MNP clusters via nuclear magnetic resonance, magnetic resonance imaging or relaxometry. On the basis of this principle, a simple detection method for lysozymes was developed.47 The presence of lysozymes provokes the destruction of aggregated MNPs, leading to an increase in T2. This sensing platform provides a relatively high detection limit of 0.5 nM.
Aptamer–nanomaterial complexes in therapy
As previously described, QDs have notable features as probe molecules. For the application of QDs to therapy, photodynamic therapy (PDT) using QDs and photosensitizers has been conducted.10 However, comparatively low apoptotic effects and lack of targeting ability have been cited as some drawbacks of PDT. Therefore, the direct delivery of drugs via aptamer-modified QDs is favored rather than PDT. The intercalating properties of several drugs into DNA or RNA aptamers allow selective and effectual therapy. The combination of drug-intercalated aptamers and QDs facilitates simultaneous imaging and therapy. For example, Bagalkot et al.37 designed a theranostic complex using aptamer-modified QDs. Doxorubicin-intercalated aptamer-QD complexes exhibited no fluorescence signal because of FRET. However, after internalization of the complex into target cells via a specific interaction between the aptamer and target surface proteins, intercalated doxorubicin was released inside the cell by lysosomes, resulting in strong fluorescence from QDs and apoptosis by doxorubicin. QD complexes can be used for the dual functions of detection probes and delivering drugs.
Photothermal therapy (PTT) has been used in cancer therapy because it is non-invasive compared with radiation therapy, chemotherapy and surgery. Using near-infrared light, photothermal agents can effectively kill target cells and minimize unwanted injury to surrounding non-target cells.48 Of the various photothermal probes, AuNPs are one of the most attractive particles because they enhance the damage induced by radiation and produce high heat upon near-infrared radiation.49 Particularly, aptamer-immobilized AuNPs are noteworthy in targeted therapy. For instance, Wang et al.50 designed a smart complex that can selectively and sensitively kill target cells via PTT, as well as via PDT. As aptamer–photosensitizer-gold nanorods (rod-shaped AuNPs) can target-specific cells using aptamers, and can also destroy cells using PTT and PDT, ultra-efficient therapy is achievable. Furthermore, multiple aptamer-modified gold nanostars (AuNSs) were also developed, as represented in Figure 6a.51 Newly synthesized AuNSs complexes showed high selectivity toward PSMA(+) and PSMA(–) cells, and extremely high efficiency for PTT using an 808-nm laser under an irradiance of 0.3 W cm−2. In addition, AuNPs can be used as drug-delivery vehicle, as mentioned previously. Luo et al.52 fabricated a drug-delivery conjugate based on AuNPs. A drug intercalated in the aptamers can be released by irradiation, leading to selective and effective therapy.
Therapeutic applications of biomolecule–nanomaterial complexes. (a) PTT using dual-aptamer-modified AuNSs. Aptamer-AuNS agents selectively induce apoptosis by generating considerable heat (A10 aptamer for PSMA (+) cancer cells and DUP1 aptamer for PSMA (–) cells). This image was adapted from Jo et al.51 (b) Aptamer-modified drug-encapsulated liposome. This image was adapted from Kang et al.54 PEG, polyethylene glycol; TMR, tetramethylrhodamine.
It is widely known that liposomes encapsulate not only hydrophilic therapeutic drugs inside the aqueous center, but also hydrophobic therapeutic drugs within the bilayer membrane.53 Studies on liposome therapy have been performed for a long time, and a few liposome-based drugs have already been approved for clinical use. The immobilization of aptamers onto the liposome surface can assign targeting capabilities to the liposome. In 2010, Kang et al.54 fabricated sgc8 aptamer-modified, liposome-targeting CEM-CCRF cells. Aptamers were immobilized on a dextran-encapsulated PEG-liposome, and then applied to CEM-CCRF cells, resulting in highly effective and selective apoptosis of target cells (Figure 6b).
In addition, micelles have also been frequently utilized in therapeutic applications and are a cluster of surfactants such as soap, phospholipids and fatty acids. In an aqueous solution, the hydrophobic tails aggregate in the center of a micelle, and the polar heads are oriented toward the outside. Therefore, targeting molecule-modified micelles can selectively deliver hydrophobic anti-cancer drugs analogous to liposome-based carriers. Mu et al.55 designed the FB4 APP (aptamer-modified PEG-polylactic acid micelles) that targets the TfR on endothelial cells in the brain. For therapy, flurbiprofen, a potential Alzheimer’s disease drug, was encapsulated inside APP molecules, and the drug complexes were applied to target cells. The micelle-based complex showed increased binding efficiency in the target brain cells.
Of the several types of magnetic particles, superparamagnetic iron oxide nanoparticles (SPIONs) possess distinct characteristics, including good dispersibility, biocompatibility and anti-biofouling properties that inhibit the adsorption of plasma proteins or cells onto their surface.56 They have been used as magnetic resonance contrast agents or magnetic field-guided drug-delivery vehicles. For the application of these molecules to therapy, drug-intercalated aptamers are immobilized on the surface of SPION in a similar manner. Thermally cross-linked SPION was modified with PSMA (+) and (−) aptamers and doxorubicin.35 According to the results, this complex can selectively and effectively release the drug to prostate cancer cells.
Concluding remarks
In this review, we have discussed a range of applications for aptamers and aptamer–nanomaterial complexes in diagnosis and therapy. The merging of aptamers and nanomaterials can provide a highly sensitive biosensing system, target-specific imaging and efficient therapy. Outstanding improvements in nanotechnology and biotechnology have generated innumerably valuable outcomes and resulted in current novel innovations; however, some challenges remain. In the case of aptamers, there are some limitations, such as a relatively short half-life, low specificity for target molecules, and exposure to serum degradation. There are also challenges with nanomaterials, including the short half-life of particles in circulation, low permeability and difficulties in large-scale synthesis. Nevertheless, aptamer–nanomaterial complexes are fascinating and have promising properties; remarkable techniques have been applied to overcome the existing weaknesses. Fervent attempts to develop innovative and creative aptamer–nanomaterial conjugates will continue, and these complexes will be recognized as novel platforms in many fields, including medical diagnostics and therapeutics.
References
Nimjee SM, Rusconi CP, Sullenger BA . Aptamers: an emerging class of therapeutics. Annu Rev Med 2005; 56: 555–583.
Carothers JM, Oestreich SC, Szostak JW . Aptamers selected for higher-affinity binding are not more specific for the target ligand. J Am Chem Soc 2006; 128: 7929–7937.
Jayasena SD . Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem 1999; 45: 1628–1650.
Proske D, Blank M, Buhmann R, Resch A . Aptamers—basic research, drug development, and clinical applications. Appl Microbiol Biotechnol 2005; 69: 367–374.
Banerjee S, Wong SS . Synthesis and characterization of carbon nanotube-nanocrystal heterostructures. Nano Lett 2002; 2: 195–200.
Pavlov V, Xiao Y, Shlyahovsky B, Willner I . Aptamer-functionalized Au nanoparticles for the amplified optical detection of thrombin. J Am Chem Soc 2004; 126: 11768–11769.
Lu AH, Salabas EL, Schuth F . Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl 2007; 46: 1222–1244.
Wang H, Yang R, Yang L, Tan W . Nucleic acid conjugated nanomaterials for enhanced molecular recognition. ACS Nano 2009; 3: 2451–2460.
Yang L, Zhang XB, Ye M, Jiang JH, Yang RH, Fu T et al. Aptamer-conjugated nanomaterials and their applications. Adv Drug Deliv Rev 2011; 63: 1361–1370.
Liu QL, Jin C, Wang YY, Fang XH, Zhang XB, Chen Z et al. Aptamer-conjugated nanomaterials for specific cancer cell recognition and targeted cancer therapy. NPG Asia Mater 2014; 6: e95.
Ellington AD, Szostak JW . In vitro selection of RNA molecules that bind specific ligands. Nature 1990; 346: 818–822.
Tuerk C, Gold L . Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990; 249: 505–510.
Levesque D, Beaudoin JD, Roy S, Perreault JP . In vitro selection and characterization of RNA aptamers binding thyroxine hormone. Biochem J 2007; 403: 129–138.
Niazi JH, Lee SJ, Gu MB . Single-stranded DNA aptamers specific for antibiotics tetracyclines. Bioorg Med Chem 2008; 16: 7245–7253.
Mendonsa SD, Bowser MT . In vitro selection of aptamers with affinity for neuropeptide Y using capillary electrophoresis. J Am Chem Soc 2005; 127: 9382–9383.
Huang CJ, Lin HI, Shiesh SC, Lee GB . Integrated microfluidic system for rapid screening of CRP aptamers utilizing systematic evolution of ligands by exponential enrichment (SELEX). Biosens Bioelectron 2010; 25: 1761–1766.
Sefah K, Shangguan D, Xiong XL, O'Donoghue MB, Tan WH . Development of DNA aptamers using Cell-SELEX. Nat Protoc 2010; 5: 1169–1185.
Darmostuk M, Rimpelova S, Gbelcova H, Ruml T . Current approaches in SELEX: an update to aptamer selection technology. Biotechnol Adv 2015; 33: 1141–1161.
Das R, Karanicolas J, Baker D . Atomic accuracy in predicting and designing noncanonical RNA structure. Nat Methods 2010; 7: 291–294.
Sarver M, Zirbel CL, Stombaugh J, Mokdad A, Leontis NB . FR3D: finding local and composite recurrent structural motifs in RNA 3D structures. J Math Biol 2008; 56: 215–252.
Jiang P, Meyer S, Hou Z, Propson NE, Soh HT, Thomson JA et al. MPBind: a Meta-motif-based statistical framework and pipeline to Predict Binding potential of SELEX-derived aptamers. Bioinformatics 2014; 30: 2665–2667.
Chushak Y, Stone MO . In silico selection of RNA aptamers. Nucleic Acids Res 2009; 37: e87.
Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I et al. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell 2011; 147: 1270–1282.
Dittmar KA, Jiang P, Park JW, Amirikian K, Wan J, Shen S et al. Genome-wide determination of a broad ESRP-regulated posttranscriptional network by high-throughput sequencing. Mol Cell Biol 2012; 32: 1468–1482.
Chen X, Zhang Q, Qian C, Hao N, Xu L, Yao C . Electrochemical aptasensor for mucin 1 based on dual signal amplification of poly(o-phenylenediamine) carrier and functionalized carbon nanotubes tracing tag. Biosens Bioelectron 2015; 64: 485–492.
Jo H, Gu H, Jeon W, Youn H, Her J, Kim SK et al. Electrochemical aptasensor of cardiac troponin i for the early diagnosis of acute myocardial infarction. Anal Chem 2015; 87: 9869–9875.
Jeon W, Lee S, Manjunatha DH, Ban C . A colorimetric aptasensor for the diagnosis of malaria based on cationic polymers and gold nanoparticles. Anal Biochem 2013; 439: 11–16.
Roncancio D, Yu H, Xu X, Wu S, Liu R, Debord J et al. A label-free aptamer-fluorophore assembly for rapid and specific detection of cocaine in biofluids. Anal Chem 2014; 86: 11100–11106.
Cella LN, Sanchez P, Zhong W, Myung NV, Chen W, Mulchandani A . Nano aptasensor for protective antigen toxin of anthrax. Anal Chem 2010; 82: 2042–2047.
Hwang KS, Lee SM, Eom K, Lee JH, Lee YS, Park JH et al. Nanomechanical microcantilever operated in vibration modes with use of RNA aptamer as receptor molecules for label-free detection of HCV helicase. Biosens Bioelectron 2007; 23: 459–465.
Bini A, Minunni M, Tombelli S, Centi S, Mascini M . Analytical performances of aptamer-based sensing for thrombin detection. Anal Chem 2007; 79: 3016–3019.
Hianik T, Ostatna V, Zajacova Z, Stoikova E, Evtugyn G . Detection of aptamer-protein interactions using QCM and electrochemical indicator methods. Bioorg Med Chem Lett 2005; 15: 291–295.
Gilbert JC, DeFeo-Fraulini T, Hutabarat RM, Horvath CJ, Merlino PG, Marsh HN et al. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation 2007; 116: 2678–2686.
Sayyed SG, Hagele H, Kulkarni OP, Endlich K, Segerer S, Eulberg D et al. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia 2009; 52: 2445–2454.
Min K, Jo H, Song K, Cho M, Chun YS, Jon S et al. Dual-aptamer-based delivery vehicle of doxorubicin to both PSMA (+) and PSMA (-) prostate cancers. Biomaterials 2011; 32: 2124–2132.
Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR et al. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol 2009; 27: 839–849.
Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R et al. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett 2007; 7: 3065–3070.
Zhou D . Quantum dot-nucleic acid/aptamer bioconjugate-based fluorimetric biosensors. Biochem Soc Trans 2012; 40: 635–639.
Zhang CY, Johnson LW . Single quantum-dot-based aptameric nanosensor for cocaine. Anal Chem 2009; 81: 3051–3055.
Liang H, Zhang XB, Lv Y, Gong L, Wang R, Zhu X et al. Functional DNA-containing nanomaterials: cellular applications in biosensing, imaging, and targeted therapy. Acc Chem Res 2014; 47: 1891–1901.
Medley CD, Smith JE, Tang Z, Wu Y, Bamrungsap S, Tan W . Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal Chem 2008; 80: 1067–1072.
Song KM, Jeong E, Jeon W, Cho M, Ban C . Aptasensor for ampicillin using gold nanoparticle based dual fluorescence-colorimetric methods. Anal Bioanal Chem 2012; 402: 2153–2161.
He XX, Wang K, Tan W, Liu B, Lin X, He C et al. Bioconjugated nanoparticles for DNA protection from cleavage. J Am Chem Soc 2003; 125: 7168–7169.
Cai L, Chen ZZ, Chen MY, Tang HW, Pang DW . MUC-1 aptamer-conjugated dye-doped silica nanoparticles for MCF-7 cells detection. Biomaterials 2013; 34: 371–381.
Jo H, Her J, Ban C . Dual aptamer-functionalized silica nanoparticles for the highly sensitive detection of breast cancer. Biosens Bioelectron 2015; 71: 129–136.
Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca EA et al. In vivo magnetic resonance imaging of transgene expression. Nat Med 2000; 6: 351–355.
Bamrungsap S, Shukoor MI, Chen T, Sefah K, Tan W . Detection of lysozyme magnetic relaxation switches based on aptamer-functionalized superparamagnetic nanoparticles. Anal Chem 2011; 83: 7795–7799.
Hesketh PJ . Chemotherapy-induced nausea and vomiting. N Engl J Med 2008; 358: 2482–2494.
Jelveh S, Chithrani DB . Gold nanostructures as a platform for combinational therapy in future cancer therapeutics. Cancers 2011; 3: 1081–1110.
Wang J, Zhu G, You M, Song E, Shukoor MI, Zhang K et al. Assembly of aptamer switch probes and photosensitizer on gold nanorods for targeted photothermal and photodynamic cancer therapy. ACS Nano 2012; 6: 5070–5077.
Jo H, Youn H, Lee S, Ban C . Ultra-effective photothermal therapy for prostate cancer cells using dual aptamer-modified gold nanostars. J Mater Chem B 2014; 2: 4862–4867.
Luo YL, Shiao YS, Huang YF . Release of photoactivatable drugs from plasmonic nanoparticles for targeted cancer therapy. ACS Nano 2011; 5: 7796–7804.
Zhou J, Rossi JJ . Cell-type-specific, aptamer-functionalized agents for targeted disease therapy. Mol Ther Nucleic Acids 2014; 3: e169.
Kang H, O'Donoghue MB, Liu H, Tan W . A liposome-based nanostructure for aptamer directed delivery. Chem Commun 2010; 46: 249–251.
Mu CF, Dave N, Hu J, Desai P, Pauletti G, Bai SH et al. Solubilization of flurbiprofen into aptamer-modified PEG-PLA micelles for targeted delivery to brain-derived endothelial cells in vitro. J Microencapsul 2013; 30: 701–708.
Lee H, Yu MK, Park S, Moon S, Min JJ, Jeong YY et al. Thermally cross-linked superparamagnetic iron oxide nanoparticles: synthesis and application as a dual imaging probe for cancer in vivo. J Am Chem Soc 2007; 129: 12739–12745.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2015027587) and a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant number: HI12C-1852-020014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Jo, H., Ban, C. Aptamer–nanoparticle complexes as powerful diagnostic and therapeutic tools. Exp Mol Med 48, e230 (2016). https://doi.org/10.1038/emm.2016.44
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2016.44
This article is cited by
-
The Application of Aptamer and Research Progress in Liver Disease
Molecular Biotechnology (2024)
-
Electrochemical aptasensor based on the engineered core-shell MOF nanostructures for the detection of tumor antigens
Journal of Nanobiotechnology (2023)
-
A novel specific aptamer targets cerebrovascular endothelial cells after ischemic stroke
Scientific Reports (2023)
-
Nanotechnology-Based Delivery of Genistein to Overcome Physicochemical Hindrance and Enhance Therapeutic Response in Skin Cancer
BioNanoScience (2023)
-
Targeted design of green carbon dot-CA-125 aptamer conjugate for the fluorescence imaging of ovarian cancer cell
Cell Biochemistry and Biophysics (2022)