Abstract
BCL-2 interacting cell death suppressor (BIS), which is ubiquitously expressed, has important roles in various cellular processes, such as apoptosis, the cellular stress response, migration and invasion and protein quality control. In particular, BIS is highly expressed in skeletal and cardiac muscles, and BIS gene mutations result in human myopathy. In this study, we show that mRNA and protein levels of BIS were markedly increased during skeletal myogenesis in C2C12 cells and mouse satellite cells. BIS knockdown did not prevent the early stage of skeletal myogenesis, but did induce muscle atrophy and a decrease in the diameter of myotubes. BIS knockdown significantly suppressed the expression level of myosin heavy chain (MyHC) without changing the expression levels of myogenic marker proteins, such as Mgn, Cav-3 and MG53. In addition, BIS endogenously interacted with MyHC, and BIS knockdown induced MyHC ubiquitination and degradation. From these data, we conclude that molecular association of MyHC and BIS is necessary for MyHC stabilization in skeletal muscle.
Similar content being viewed by others
Introduction
Skeletal and cardiac muscles are dynamic organs that are constantly adapting in response to mechanical loading and metabolic changes. Autophagosomal and proteasomal protein degradation is critical to ensure muscle integrity and mass; these processes mediate protein quality control by removing damaged proteins and organelles.1, 2 There is an association between the dysfunctional regulation of these pathways and various muscular diseases.2 Myofibrillar myopathy (MFM) represents a group of muscular dystrophies with progressive muscle weakness, which is morphologically characterized by the disintegration of the z-disk structure and the intracellular accumulation of degradation products.3, 4 Mutations in at least nine genes have been found in one MFM patient group. Of these genes, mutations in BCL-2 interacting cell death suppressor (BIS) lead to severe MFM.5, 6, 7, 8
The BIS protein was originally identified as a BCL-2 binding protein that enhances the anti-apoptotic activity of BCL-2.9 Because of its ability to bind to various proteins, such as BCL-2, HSP70, HSPB8 and MMP2, BIS is involved in multiple cellular processes, including apoptosis, stress responses, macroautophagy, chaperone-assisted selective autophagy, cell adhesion and migration and senescence.9, 10, 11, 12, 13, 14, 15, 16, 17 BIS is ubiquitously expressed with the highest levels of expression occurring in skeletal and cardiac muscle.9, 18 Two research groups have observed different skeletal muscle phenotypes in Bis-disrupted mice. Homma et al. have reported that Bis disruption results in massive apoptosis in cardiac and skeletal muscle and we have observed a decrease in myofibril mass with distinctly disrupted z-disk structure. These data suggest that insufficient quantities of BIS appear to be associated with muscular dysfunction, which is in accord with the identification of BIS gene mutations in MFM patients.
BIS protects the actin-capping protein CapZ from proteasomal degradation and stimulates the clearance of damaged filamin C via chaperone-assisted selective autophagy.16, 19, 20 Ruparelia et al.21 have demonstrated that BIS depletion results in myofibrillar disintegration without protein aggregation, whereas the overexpression of the P209L BIS mutant leads to the formation of protein aggregates without myofibrillar disintegration. Furthermore, protein degradation induced by the P209L BIS mutation is not substantially inhibited by autophagy stimulation. Thus, it is possible that BIS coordinates muscle integrity through an autophagy-independent pathway that targets other z-disk structural proteins. Because MFM is caused by protein aggregation and myofibrillar disintegration, and because BIS is involved in the selective clearance of misfolded proteins, there might be a possible link between the MFM phenotype and BIS gene mutation.
Myosin heavy chain (MyHC), which constitutes sarcomere thick filaments, functions as a molecular motor protein in skeletal and cardiac muscle. MyHC mutations have also been found in various human MFM patients. Notably, different mutations in MyHC isoforms (MYH2, MYH3, MYH6, MYH7 and MYH8) lead to various skeletal muscle diseases, such as hypertrophic and dilated cardiomyopathies.22, 23 Because mutations in BIS and MyHC isoforms are found in human MFM patients, there might be a correlation between BIS and MyHC. In the present study, we report that the mRNA and protein expression levels of BIS gradually increased during skeletal myogenesis. BIS knockdown resulted in a significant decrease in MyHC expression accompanied by a decrease in myotube diameter. Our results suggest that the molecular association of BIS with MyHC prevents MyHC ubiquitination and degradation, thereby helping to maintain the myofibril mass in skeletal muscle.
Materials and methods
Reagents and antibodies
Bafilomycin A1 was purchased from Sigma-Aldrich (St Louis, MO, USA). MG132 was purchased from Selleckchem (Houston, TX, USA). The following antibodies were used for immunoblot or immunofluorescence experiments. Antibodies specific for MuRF1, Myf-5, Myf-6, α-actin and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies for Mgn, Cav-3 and MyoD were purchased from BD Biosciences (San Jose, CA, USA). Anti-LC3B and anti-Ub antibodies were provided by Cell Signaling Technology (Danvers, MA, USA). Anti-MyHC and Atrogin-1 antibodies were obtained from Sigma-Aldrich and ECM Biosciences (Versailles, KY, USA), respectively. The anti-serum against BIS or MG53 was prepared as previously described.9, 24
Cell culture and isolation of primary myoblasts
C2C12 cells were purchased from ATCC (Manassas, VA, USA) and grown in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 1% penicillin/streptomycin (Thermo Fisher Scientific) and 10% fetal bovine serum (Thermo Fisher Scientific) in a 5% CO2 incubator at 37 °C. Confluent C2C12 cells were differentiated into myotubes by incubation in differentiation medium with 2% horse serum (Thermo Fisher Scientific) and feeding every 24 h.
To prepare primary myoblasts, 8- to 12-week-old C57BL/7 mice or Bisflox/− mice were injected with 0.1 mg ml−1 cardiotoxin from Naja mossambica (Sigma-Aldrich) in the tibialis anterior and quadriceps. After 3 days, the tibialis anterior and quadriceps were dissected from mice and incubated in 250 CDU per ml of collagenase from Clostridium histolyticum (Sigma-Aldrich) in DMEM for 30 min at 37 °C. The muscle fiber suspension was transferred to 100 mm culture dishes, and the collagenase solution was removed. The muscle was washed with Dulbecco’s phosphate-buffered saline (DPBS) and triturated with the broken end of a 5-ml Pasteur pipet in 5 ml of growth media composed of Ham’s F10 medium (Thermo Fisher Scientific) containing 20% horse serum, 5 ng ml−1 FGF basic (R&D Systems, Minneapolis, MN, USA), and 1% penicillin/streptomycin. To concentrate the muscle fibers, the culture dish was tilted on the side, and the muscle fibers were transferred to a conical tube. Five milliliters of growth medium was added to the culture dish. The muscle was triturated, collected in a conical tube and centrifuged at 300 g for 3 min at room temperature. The supernatant was discarded and the pellet was washed with DPBS, re-suspended in growth medium and plated on 100 mm Matrigel-coated culture dishes (Corning Life Science, Corning, NY, USA). If necessary, BIS knockout was performed by infecting the Cre-expressing adenovirus, as described below. To differentiate myotubes, cells were incubated in differentiation medium for 2 days.
BIS knockdown and BIS knockout
BIS knockdown in C2C12 cells was performed by siRNA oligomers targeting BIS (5′-AAGGUUCAGACCAUCUUGGAA-3′) and a scrambled oligomer (5′-CCUACGCCACCAAUUUCGUdTdT-3′) (Bioneer, Daejeon, Korea) using electroporation (MP-100, Thermo Fisher Scientific). BIS-knockout primary myoblasts were generated with the infection of adenovirus encoding Cre into the myoblasts from Bisflox/− mice. To prepare Bisflox/− mice, the germ line-transmitted male mice from chimeric mice with the targeted allele25 were mated with EIIa-cre C57B6 female mice (The Jackson Laboratory, Bar Harbor, ME, USA). The male offspring that harbored various types of deletions in the targeting allele were mated with wild C57B6 females to obtain the germ line-transmitted mice with Bisflox in one allele. To ensure the BIS deletion effect, Bisflox/+ mice were mated with Bis heterozygotes to obtain Bisflox/− mice. Primary myoblasts isolated from Bisflox/− mice were plated on cell-culture dishes. At 24 h after the cell seeding, the cells were infected by adenoviruses encoding LacZ, GFP or Cre24 at 9 multiplicity of infection for 16 h. Cells were stabilized with growth medium for 12 h, followed by differentiation to myotubes.
Immunoblot
Cells were lysed with an RIPA lysis buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 2 mM EDTA) supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktail (Roche, Indianapolis, IN, USA). After centrifugation at 14 000 r.p.m. for 10 min at 4 °C, the whole cell lysates (supernatant) were separated in SDS-polyacrylamide gels and transferred onto a NC membrane. Antigens were visualized by sequential treatment with specific antibodies, HRP-conjugated secondary antibodies (Thermo Fisher Scientific) and an enhanced chemiluminescence substrate kit (Thermo Fisher Scientific).
Immunofluorescence
C2C12 cells or muscle fibers were fixed with 4% paraformaldehyde in DPBS for 10 min, permeabilized with 0.2% TX-100 for 10 min and incubated with blocking buffer (2% BSA in DPBS). Samples were incubated for 1 h with primary antibodies. After three washes in DPBS for 5 min, samples were stained for 1 h with fluorescein-conjugated secondary antibodies followed by DAPI staining for 5 min and were then observed with a LSM 700 confocal microscope (Carl Zeiss Jena GmbH, Jena, Germany) or an epifluorescence microscope (Nikon Corporation, Tokyo, Japan). After co-staining with anti-MyHC antibody and DAPI (Thermo Fisher Scientific), the myogenic index was determined as the average number of nuclei from the MyHC-positive myotubes in five separate images as previously described.26
A single muscle fiber was isolated as follows. The tibialis anterior and quadriceps were dissected from mice and incubated in 250 CDU per ml of collagenase from Clostridium histolyticum (Sigma-Aldrich) in DMEM for 30 min at 37 °C. The collagenase solution was removed from the muscle. The muscle tissues were washed with DPBS, and a single muscle fiber was released by flushing the muscle fiber using a Pasteur pipet in DMEM. The presence of a single muscle fiber was confirmed under a light microscope and then analyzed by immunofluorescence.
Quantitative real-time PCR
Total RNA was extracted from cultured cells with a RNA extraction kit (iNtRON Biotechnology, Gyeonggi-do, Korea), and cDNA was synthesized using PrimeScript RTase (Clontech Takara Bio., Mountain View, CA, USA). Gene expression was measured according to standard quantitative PCR (qPCR) procedures with ToPreal qPCR 2X PreMIX (Enzynomics, Daejeon, Korea). A LightCycler 480 Instrument II (Roche) was used with following primers: BIS-F (5′-ACTCTAAGCCTGTTTCCCAGAAGT-3′), BIS-R (5′-AGACTTGTACTTGACCTGGGACAT-3′), MyHC1-F (5′-CAAGCAGCAGTTGGATGAGCGACT-3′), MyHC1-R (5′-TCCTCCAGCTCCTCGATGCGT-3′), MyHC2a-F (5′-AGAGGACGACTGCAGACCGAAT-3′), MyHC2a-R (5′-GAGTGAATGCTTGCTTCCCCCTTG-3′), MyHC2b-F (5′-ACGCTTGCACACAGAGTCAG-3′), MyHC2b-R (5′-CTTGGACTCTTCCTCTAGCTGCC-3′), MyHC2x-F (5′-ACCAAGGAGGAGGAACAGCAGC-3′), MyHC2x-R (5′-GAATGCCTGTTTGCCCCTGGAG-3′), Atrogin-1-F (5′-CTCTGTACCATGCCGTTCCT-3′), Atrogin-1-R (5′-GGCTGCTGAACAGATTCTCC-3′), MuRF1-F (5′-GACAGTCGCATTTCAAAGCA-3′) and MuRF1-R (5′-AACGACCTCCAGACATGGAC-3′). Expression amounts were normalized to 18S or β-actin and represented as fold change.
Luciferase assay
The BIS promoter–luciferase reporter constructs were previously generated.27 After transfection of these constructs to C2C12 myoblasts or primary myoblasts by electroporation, the cells were differentiated into myotubes. The luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) and GloMax 20/20 Single Tube Luminometer (Promega), which was normalized to Renilla activity.
Pulse-chase analysis
Pulse-chase analysis was performed as previously described.28 Briefly, C2C12 myoblasts were transfected with si-control or si-BIS (100 nM) and differentiated for 4 days. The myotubes were incubated with methionine- and cysteine-free DMEM (Sigma-Aldrich) for 1 h, pulsed with 10 μCi ml−1 of EasyTag EXPRESS35S Protein Labeling Mix (Perkin-Elmer, Waltham, MA, USA) for 2 h and then chased with DMEM-containing 2% horse serum for the indicated times. Total cell lysates were immunoprecipitated with an anti-MyHC antibody and separated by SDS–PAGE. 35S-labeled MyHC was visualized by autoradiography. The intensity of 35S-labeled MyHC radiation was assessed using the Quantity One program (Bio-Rad, Hercules, CA, USA), normalized to β-actin, and represented as the remaining MyHC rate.
Immunoprecipitation
The cells were lysed in a buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 2 mM EDTA, 1 mM EGTA, 0.5 mM DTT and a protease inhibitor cocktail (Roche). The whole-cell lysates were centrifuged at 1400 r.p.m. for 10 min at 4 °C; this was followed by pre-clearing with protein A or protein G agarose beads for 1 h at 4 °C. The supernatant (1–2 mg of total protein) was incubated with specific antibodies for BIS or MyHC (1–3 μg) overnight and then with 30 μl of a protein A or protein G agarose bead slurry (Roche) for 3 h at 4 °C. To elute the proteins from the beads, the beads were washed with lysis buffer three times and boiled in 2 × SDS-protein sample buffer (100 mM Tris-HCl, pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% glycerol, 200 mM DTT) for 5 min at 100 °C. The protein samples were assessed by immunoblot for α-actin, BIS or MyHC.
Statistical analysis
At least three replicates were analyzed for each experiment presented. Statistical values are presented as the mean±s.e. A two-tailed Student’s t-test was used to calculate the P-values. P-values <0.05 were considered as statistically significant.
Results
BIS protein is increased during skeletal myogenesis
To understand the role of BIS in skeletal myogenesis, we analyzed BIS protein and mRNA levels by immunoblot and qPCR during skeletal myogenesis of C2C12 cells. As shown in Figure 1a, BIS protein levels gradually increased along with myogenic marker proteins, such as MyHC, Mitsugumin 53 (MG53), Myogenin (Mgn) and Caveolin-3 (Cav-3). qPCR analysis also showed that the mRNA levels of BIS, MyHC1 and MyHC2b were significantly increased compared with those of C2C12 myoblasts (Figure 1b). Next, we measured luciferase activity in C2C12 myoblasts and myotubes after the transient expression of a luciferase reporter gene under control of the BIS gene promoter. C2C12 myotubes showed a 4.3-fold increase in luciferase activity compared with that of C2C12 myoblasts (Figure 1c).
The expression profile of BCL-2 interacting cell death suppressor (BIS) during myogenic differentiation. (a–c) C2C12 myoblasts were differentiated into myotubes for the indicated number of days. MB denotes myoblasts. MT denotes 4-day-differentiated myotubes. (d–f) Mouse satellite cells were differentiated into myotubes for the indicated number of days. MB denotes myoblasts. MT denotes 2-day-differentiated myotubes. (a, d) The expression levels of BIS and myogenic marker proteins, such as myosin heavy chain (MyHC), mitsugumin 53 (MG53), myogenin (Mgn) and caveolin-3 (Cav-3), were assessed by immunoblotting. β-Actin was used as a loading control. (b, e) Quantitative real-time PCR (qPCR) was used for analyzing the relative expression of BIS, MyHC1 and MyHC2b mRNAs (n=3). (c) C2C12 myoblasts were transiently transfected with pRL vector or a BIS promoter construct (−1080 to +289) that were cloned into the pGL3-basic vector and differentiated for 3 days. Luciferase reporter activity was measured and calculated as firefly RLU/Renilla RLU in MB and MT (n=3). RLU is defined as relative luciferase units. (f) Luciferase reporter activity was measured in 2-day-differentiated myotubes of mouse satellite cells and C2C12 cells (n=3). *P<0.05, **P<0.01, ***P<0.001 relative to MB.
Next, we examined BIS induction during skeletal myogenesis in mouse satellite cells, which are primary mouse myoblasts. BIS protein levels were greatly increased, as were other myogenic markers (MyHC, MG53, Mgn and Cav-3), during myogenesis (Figure 1d). In addition, the BIS mRNA level was 1.7-fold higher in satellite cell-derived myotubes than in undifferentiated satellite cells (Figure 1e). Satellite cell-derived myotubes exhibit a decrease in MyHC1, an isoform expressed in slow twitch muscle fiber, which might be attributable to the source of the satellite cells that were isolated from fast type muscles such as tibialis and quadriceps muscles. A reporter assay for BIS promoter activity also confirmed the transcriptional activation of BIS during satellite cell myogenesis (Figure 1f).
BIS knockdown decreases MyHC protein level
BIS protein induction during skeletal myogenesis led us to investigate the role of BIS in muscle differentiation. We monitored C2C12 myogenesis by MyHC and MG53 immunofluorescence after BIS knockdown. It should be noted that MG53 is a critical myogenic marker protein.24, 28, 29 As shown in Figure 2a, the MyHC immunofluorescence signal was prominently weaker in si-BIS-treated myotubes than in si-control-treated myotubes, whereas the MG53 immunofluorescence signal was not different between si-BIS and si-control-treated myotubes. We also measured the myogenic index and the diameter of myotubes after BIS knockdown. Figures 2b and c show that the myogenic index and diameter were lower in BIS knockdown myotubes than in si-control myotubes. Immunoblot analysis also showed that BIS knockdown resulted in a considerable decrease in MyHC protein levels, but did not change the expression level of other myogenic differentiation markers (MG53, Mgn and Cav-3) (Figure 2d). These data indicate that BIS knockdown induced muscular atrophy in C2C12 cells by reducing the expression of MyHC protein.
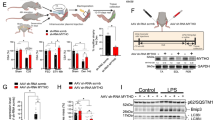
The effect of BCL-2 interacting cell death suppressor (BIS) knockdown on myogenic differentiation. (a–d) C2C12 myoblasts were transfected with 100 nM of si-control (si-CON) or si-BIS and differentiated into myotubes for 4 days. (e–h) Satellite cells were obtained from Bisflox/− mice, infected with adenovirus encoding LacZ (Ad-LacZ) or Cre (Ad-Cre) and differentiated into myotubes for 2 days. C2C12- (a) and satellite- (e) derived myotubes were immunostained for myosin heavy chain (MyHC) or MG53. Nuclei were counterstained with DAPI. Scale bar=100 μm. (b, f) The myogenic index was calculated as the number of nuclei in MyHC-stained myotubes (n=5). (c, g) The diameter of myotubes was assessed from MG53-stained myotubes (n=5). (d, h) The expression level of BIS and myogenic marker proteins (MyHC, MG53, Mgn and Cav-3) was assessed by immunoblotting. (i) C2C12 myoblasts were transfected with 100 nM si-CON or si-BIS, further incubated in differentiation media for 12 h and immunostained for BIS and myogenic transcription factors (MyoD, Myf-5, Myf-6 and Mgn). Nuclei were counterstained with DAPI. Scale bar=50 μm. **P<0.01, ***P<0.001 relative to si-CON or Ad-LacZ.
Next, satellite cells were isolated from the gastrocnemius muscles of Bisflox/− mice, infected with adenovirus containing Cre recombinase to suppress BIS expression and differentiated into myotubes. The myogenesis of satellite cells was monitored by MyHC and MG53 immunostaining, the myogenic index, the myotube diameter and immunoblotting (Figures 2e–h). Compared with LacZ-infected cells, Cre-infected myotubes revealed a decrease in the myogenic index and the myotube diameter (Figures 2e–g). Similarly to the results in C2C12 myotubes, BIS knockdown decreased the expression level of MyHC without changing the expression level of other myogenic markers (MG53, Mgn and Cav-3) (Figure 2h).
Next, we determined whether BIS knockdown affects the activation of myogenic transcription factors, such as MyoD, Mgn, Myf5 and Myf6 during C2C12 myogenesis. It should be noted that these transcription factors are found in the nuclei in the early stage of skeletal myogenesis. As shown by immunofluorescence (Figure 2i), BIS knockdown did not change the nuclear localization of any of the myogenic transcription factors, suggesting that BIS is not involved in the early stage of skeletal myogenesis.
BIS is necessary for MyHC stability
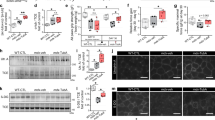
As quantified in the immunoblot data of Figure 2d, the MyHC protein level was reduced to 23% in BIS-knockdown C2C12 myotubes, compared with si-control cells (Figure 3a). To determine whether BIS regulates the transcriptional regulation of MyHC, we measured the mRNA level of MyHC isoforms by qPCR after BIS knockdown in C2C12 myotubes. As shown in Figure 3b, mRNA levels of MyHC1, MyHC2a, MyHC2b and MyHC2x were slightly decreased after BIS knockdown, but there was no significant difference, suggesting that BIS knockdown regulates MyHC expression at the post-translational level. Thus, we performed pulse-chase experiments in si-control or si-BIS-treated C2C12 myotubes to address whether MyHC protein stability is affected by BIS knockdown. Figure 3c shows that [35S]-radiolabeled MyHC protein was more rapidly degraded in si-BIS-treated C2C12 myotubes than in si-control-treated cells. These data indicate that BIS is necessary for the prevention of MyHC degradation.
BCL-2 interacting cell death suppressor (BIS) knockdown decreases myosin heavy chain (MyHC) protein stability. C2C12 myoblasts were transfected with 100 nM of si-CON or si-BIS and differentiated into myotubes for 4 days. (a) Densitometric analysis of the MyHC/β-actin ratio, in reference to Figure 2d (n=3). (b) Quantitative PCR (qPCR) for the expression analysis of MyHC isoforms (n=3). (c) Stability of MyHC was determined by pulse-chase experiments using [35S]-labeled L-methionine and L-cysteine. A representative autoradiograph following immunoprecipitation with anti-MyHC antibody is shown in the left panel. The mean values from quantitative analysis are shown in the graph on the right (n=3). (d, e) C2C12 myotubes were treated with 1 μM MG132 (d) or 200 nM BafA1 (e) for the indicated times. MyHC and BIS expression levels were determined by immunoblotting. The graphs on the right show the relative ratio of MyHC/β-actin (n=3). *P<0.05, **P<0.01, ***P<0.001 relative to si-CON.
We then examined whether MyHC degradation is proteasome- or autophagosome-dependent by using BIS knockdown C2C12 myotubes treated with the proteasome inhibitor MG132 or the autophagy inhibitor Bafilomycin A1 (BafA1). As shown in Figures 3d and e, BIS expression levels were restored in the presence of MG132 but not BafA1, indicating that BIS knockdown induced the proteasomal degradation of MyHC, leading to a decreased level of MyHC protein.
To confirm the involvement of the ubiquitin-proteasome system in BIS knockdown-induced MyHC degradation, MyHC ubiquitination was determined by ubiquitin immunoblotting after MyHC immunoprecipitation in BIS-knockdown C2C12 myotubes. Figure 4a showed that BIS knockdown induced MyHC ubiquitination, which was enhanced by MG132 treatment. However, BIS knockdown did not change the expression levels of MuRF1 or Atrogin-1 (Figures 4b and c), which are the major ubiquitin ligases in atrophic skeletal muscle30, indicating that the BIS knockdown-induced MyHC ubiquitination and degradation is independent from the activation of MuRF1 and Atrogin-1.
BCL-2 interacting cell death suppressor (BIS) regulates proteasomal degradation of myosin heavy chain (MyHC) via protein–protein interaction. (a) C2C12 myoblasts were transfected with 100 nM of si-CON or si-BIS, differentiated into myotubes for 4 days, and incubated with 1 μM of MG132 for 6 h. MyHC ubiquitination was analyzed using MyHC immunoprecipitation followed by ubiquitin (Ub) immunoblotting. (b, c) Atrogin-1 and MuRF1 expression levels were determined by immunoblotting (b) and quantitative PCR (qPCR) analysis (c) in the differentiated C2C12 cells treated with si-CON or si-BIS (n=3).
BIS interacts with MyHC
Because BIS knockdown induced MyHC ubiquitination and degradation, it is tempting to speculate that BIS physically interacts with MyHC, thereby forming a relatively stable complex. To address this issue, we determined the cellular localization of MyHC and BIS by immunofluorescence in mouse gastrocnemius muscles. As shown in Figure 5a, the two proteins were colocalized, which was comparable to the previously reported colocalization of BIS and α-actin.15 Furthermore, co-immunoprecipitation assays showed that BIS physically interacted with MyHC in C2C12 myotubes (Figure 5b). Together, these results indicate that BIS is necessary for MyHC stabilization in skeletal muscle, which prevents the proteasomal degradation of MyHC.
BCL-2 interacting cell death suppressor (BIS) interacts with myosin heavy chain (MyHC). (a) BIS was co-stained along with MyHC or α-actin in single muscle fibers from gastrocnemius muscles. Scale bar=10 μm. (b) Molecular interaction between BIS and MyHC was determined by reciprocal co-immunoprecipitation for endogenous BIS and MyHC in 4-day-differentiated C2C12 myotubes.
Discussion
Because BIS is highly expressed in skeletal muscles and because BIS gene mutations result in the development of human MFM, BIS might have a physiological role in skeletal muscle. In the present study, we found that BIS protein levels were gradually increased during skeletal myogenesis in C2C12 cells and mouse satellite cells. BIS knockdown-induced MyHC ubiquitination and degradation and the molecular association of BIS with MyHC indicate that BIS is essential for MyHC stabilization in skeletal muscle.
Homma et al.18 have argued that BIS knockdown prevents skeletal myogenesis because BIS-knockdown C2C12 myotubes have a lower percentage of multinucleated cells among desmin-positive cells and increased apoptosis compared with si-control cells. We also observed that BIS knockdown in C2C12 cells decreased the fusion index to a lesser extent than what was observed in previous findings. However, our current data show that BIS knockdown did not alter the nuclear localization of myogenic transcription factors (MyoD, Mgn, Myf5 and Myf7) or the expression level of myogenic markers (Mgn, Cav-3 and MG53) during skeletal myogenesis in C2C12 cells or mouse satellite cells, which was not mentioned in the previous study (Figure 2). The results from our elaborate experiments for skeletal myogenesis led to the conclusion that BIS is not involved in skeletal myogenesis at an early stage. However, BIS knockdown led to a significant decrease in myotube diameter and MyHC expression (Figure 2), suggesting that BIS knockdown induces skeletal muscle atrophy independently of the expression of MuRF-1 and atrogin-1. This suggestion is in agreement with skeletal muscle phenotypes of Bis-disrupted mice, including notable decreases in myofibril mass with z-disk disruption and eccentric localization of myofibril nuclei representing mature myocytes.25 Furthermore, the observation that BIS knockdown induced significant atrophy in the myotubes of satellite cells (Figures 2e and g) suggests the importance of BIS in the regeneration or repair of myofibrils following physical insult or denervation as well as in normal conditions.
Homeostasis in muscle mass is tightly regulated by a coordinated balance between protein degradation and synthesis within muscle fibers according to physiological and pathological conditions.1, 2 Two major degradation pathways, the autophagy-lysosome system and the ubiquitin-proteasome system, are involved in myofibrillar protein degradation, leading to a net loss of proteins and organelles and subsequently to the shrinkage of myofibers.1 We demonstrated that BIS knockdown decreased MyHC stability via ubiquitin-proteasome degradation but not by the autophagy-lysosome system, based on the recovery of MyHC levels by a proteasome inhibitor but not by an autophagy inhibitor (Figure 3). Because BIS physically interacted with MyHC (Figures 4 and 5), the molecular association of MyHC with BIS might block the access of MyHC to ubiquitin-proteasome, leading to MyHC stabilization.
The role of BIS in the maintenance of muscle integrity has been previously suggested with a focus on the structural stability of F-actin.19 BIS does not directly bind to CapZB1, an actin capping protein, but it promotes the interaction between Hsc70 and CapZB1, resulting in a tight association of CapZB1 with F-actin at the z-disk in cardiomyocytes. In the absence of BIS, CapZB1 is destabilized and subsequently degraded through ubiquitin-proteasome machinery because CapZB1 fails to localize to the z-disc. MyHC might have a similar fate as CapZB1 in the absence of BIS.
We showed that BIS knockdown resulted in muscular atrophy, which was probably because of the acceleration of the proteasomal degradation of MyHC, suggesting a novel and essential role for BIS in the maintenance of muscle mass through MyHC stabilization. These findings support the phenotypes in Bis-disrupted mice and MFM human patients with progressive atrophy and muscle weakness, raising the possibility of targeting the BIS gene as a therapy for MFM. However, further experiments are needed to elucidate the molecular mechanism by which BIS protects MyHC from proteasomal degradation.
References
Sandri M . Autophagy in skeletal muscle. FEBS Lett 2010; 584: 1411–1416.
Sandri M, Coletto L, Grumati P, Bonaldo P . Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J Cell Sci 2013; 126: 5325–5333.
Olive M, Kley RA, Goldfarb LG . Myofibrillar myopathies: new developments. Curr. Opin. Neurol. 2013; 26: 527–535.
Selcen D, Engel AG . Myofibrillar myopathies. Handb Clin Neurol 2011; 101: 143–154.
Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV et al. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol 2009; 65: 83–89.
Odgerel Z, Sarkozy A, Lee HS, McKenna C, Rankin J, Straub V et al. Inheritance patterns and phenotypic features of myofibrillar myopathy associated with a BAG3 mutation. Neuromuscul Disord 2010; 20: 438–442.
Semmler AL, Sacconi S, Bach JE, Liebe C, Burmann J, Kley RA et al. Unusual multisystemic involvement and a novel BAG3 mutation revealed by NGS screening in a large cohort of myofibrillar myopathies. Orphanet J Rare Dis 2014; 9: 121.
Konersman CG, Bordini BJ, Scharer G, Lawlor MW, Zangwill S, Southern JF et al. BAG3 myofibrillar myopathy presenting with cardiomyopathy. Neuromuscul Disord 2015; 25: 418–422.
Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y . Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene 1999; 18: 6183–6190.
Takayama S, Xie Z, Reed JC . An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem 1999; 274: 781–786.
Carra S, Seguin SJ, Lambert H, Landry J . HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem 2008; 283: 1437–1444.
Suzuki M, Iwasaki M, Sugio A, Hishiya A, Tanaka R, Endo T et al. BAG3 (BCL2-associated athanogene 3) interacts with MMP-2 to positively regulate invasion by ovarian carcinoma cells. Cancer Lett 2011; 303: 65–71.
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C . Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 2009; 28: 889–901.
Kassis JN, Guancial EA, Doong H, Virador V, Kohn EC . CAIR-1/BAG-3 modulates cell adhesion and migration by downregulating activity of focal adhesion proteins. Exp Cell Res 2006; 312: 2962–2971.
Iwasaki M, Homma S, Hishiya A, Dolezal SJ, Reed JC, Takayama S . BAG3 regulates motility and adhesion of epithelial cancer cells. Cancer Res 2007; 67: 10252–10259.
Ulbricht A, Eppler FJ, Tapia VE, van der Ven PF, Hampe N, Hersch N et al. Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr Biol 2013; 23: 430–435.
Lee JJ, Lee JS, Cui MN, Yun HH, Kim HY, Lee SH et al. BIS targeting induces cellular senescence through the regulation of 14-3-3 zeta/STAT3/SKP2/p27 in glioblastoma cells. Cell Death Dis 2014; 5: e1537.
Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, Takayama S . BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol 2006; 169: 761–773.
Hishiya A, Kitazawa T, Takayama S . BAG3 and Hsc70 interact with actin capping protein CapZ to maintain myofibrillar integrity under mechanical stress. Circ Res 2010; 107: 1220–1231.
Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M et al. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol 2010; 20: 143–148.
Ruparelia AA, Oorschot V, Vaz R, Ramm G, Bryson-Richardson RJ . Zebrafish models of BAG3 myofibrillar myopathy suggest a toxic gain of function leading to BAG3 insufficiency. Acta Neuropathol 2014; 128: 821–833.
Martinsson T, Oldfors A, Darin N, Berg K, Tajsharghi H, Kyllerman M et al. Autosomal dominant myopathy: missense mutation (Glu-706 —> Lys) in the myosin heavy chain IIa gene. Proc Natl Acad Sci USA 2000; 97: 14614–14619.
Tajsharghi H, Oldfors A . Myosinopathies: pathology and mechanisms. Acta Neuropathol 2013; 125: 3–18.
Lee CS, Yi JS, Jung SY, Kim BW, Lee NR, Choo HJ et al. TRIM72 negatively regulates myogenesis via targeting insulin receptor substrate-1. Cell Death Differ 2010; 17: 1254–1265.
Youn DY, Lee DH, Lim MH, Yoon JS, Lim JH, Jung SE et al. Bis deficiency results in early lethality with metabolic deterioration and involution of spleen and thymus. Am J Physiol Endocrinol. Metab. 2008; 295: E1349–E1357.
Hong J, Kim BW, Choo HJ, Park JJ, Yi JS, Yu DM et al. Mitochondrial complex I deficiency enhances skeletal myogenesis but impairs insulin signaling through SIRT1 inactivation. J Biol Chem 2014; 289: 20012–20025.
Yoo HJ, Im CN, Youn DY, Yun HH, Lee JH . Bis is induced by oxidative stress via activation of HSF1. Korean J Physiol Pharmacol 2014; 18: 403–409.
Yi JS, Park JS, Ham YM, Nguyen N, Lee NR, Hong J et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat Commun 2013; 4: 2354.
Jung SY, Ko YG . TRIM72, a novel negative feedback regulator of myogenesis, is transcriptionally activated by the synergism of MyoD (or myogenin) and MEF2. Biochem Biophys Res Commun 2010; 396: 238–245.
Bodine SC, Baehr LM . Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab 2014; 307: E469–E484.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the minister of Education, Science and Technology (2011-001752, 2015R1A5A1009024 and 2012R1A1A2007589).
Author contributions
YGK and JHL developed the concept of the research, interpreted the results and edited the manuscript. JH, JSP, HL, JMJ, HYK and HHY performed the experiments. JH also contributed to the drafting of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hong, J., Park, JS., Lee, H. et al. Myosin heavy chain is stabilized by BCL-2 interacting cell death suppressor (BIS) in skeletal muscle. Exp Mol Med 48, e225 (2016). https://doi.org/10.1038/emm.2016.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2016.2
This article is cited by
-
High-intensity interval training alleviates exhaustive exercise-induced HSP70-assisted selective autophagy in skeletal muscle
The Journal of Physiological Sciences (2023)
-
Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering
BMC Cell Biology (2017)
-
Fine-tuning of actin dynamics by the HSPB8-BAG3 chaperone complex facilitates cytokinesis and contributes to its impact on cell division
Cell Stress and Chaperones (2017)
-
BIS overexpression does not affect the sensitivity of HEK 293T cells against apoptosis
Molecular & Cellular Toxicology (2017)