Abstract
Triptolide, a compound extracted from the traditional Chinese medicine preparation of Tripterygium wilfordii Hook F., has been reported to have anti-inflammatory and anti-cancer activities. However, its effect on ovarian cancer invasion is unknown. We observed that MMP7 and MMP19 expression increased in ovarian cancer tissue. Triptolide treatment inhibited the migration and invasion of ovarian cancer cells SKOV3 and A2780 at the concentration of 15 nM. We also observed that triptolide suppressed MMP7 and MMP19 promoter activity in a dose-dependent manner, down-regulating the expressions of these promoters on mRNA and protein level. Moreover, triptolide enhanced E-cadherin expression in ovarian cancer cells. In vivo, triptolide inhibited tumor formation and metastasis in nude mice, and suppressed MMP7 and MMP19 expression; it also enhanced E-cadherin expression in tumor in a dose-dependent manner. Over expression of MMP7 and MMP19, or suppression of E-cadherin expression partially abolished the inhibitory effect of triptolide on invasion of ovarian cancer cells. To summarize, triptolide significantly inhibited the migration and invasion of ovarian cancer cells by suppression of MMP7 and MMP19 and up-regulation of E-cadherin expression. This study shows that triptolide is a good candidate for the treatment of ovarian cancer and reduction of metastasis.
Similar content being viewed by others
Introduction
Triptolide is a compound originally extracted from the traditional Chinese medicinal preparation of Tripterygium wilfordii Hook F. With its broad-spectrum anticancer activity, triptolide has a considerable potential as chemotherapeutical agent. It inhibits colon cancer, breast cancer, renal cell carcinoma, and cervical carcinogenesis process (Wang et al., 2009; Kim et al., 2010; Li et al., 2011; Tan et al., 2011). Recently, it has been found to be effective in the treatment of ovarian cancer (Zhou et al., 2010); it has been shown that triptolide increases the proportion of cells in the S-phase of the cell cycle and induces apoptosis. Numerous biological molecules inhibited by triptolide have been viewed as its possible targets. Triptolide changes the expression of cell cycle regulators, apoptosis-related factors and cell proliferation markers; for example, it up-regulates LRAP, CDH4, and SFRP1 and down-regulates the expression of cystatin, TNNT 1, and L1-CAM (Westfall et al., 2008; Li et al., 2010). However, the effects of triptolide on ovarian cancer cell invasion have not been investigated.
Its high invasion and metastatic potential make ovarian cancer a particularly life-threatening disease and affect the efficacy of chemotherapy treatment. Invasion and migration of cancer cells are complicated processes in which many types of molecules are involved. Rho GTPases, such as Rac (Wang et al., 2009), RhoA (Jackson et al., 2011), and Cdc42 (Du et al., 2009) play crucial roles in cytoskeleton reorganization, adhesion and motility. Degradation of extracellular matrix (ECM) is an important step in cancer invasion into neighboring organs and initiation of metastasis. Matrix metalloproteinases (MMPs) have been implicated in cell motility and invasion by the virtue of their ability to degrade ECM components. Induced expression or overexpression of MMPs is observed in the pathogenesis of diverse diseases, such as periodontitis, rheumatoid arthritis, and skin wound healing (Vu and Werb, 2000; Sorsa et al., 2004; Kanbe et al., 2011). Moreover, MMPs have been found to play a crucial role in the development of many cancers. MMPs promote the epithelial-to-mesenchymal transition (EMT) associated with malignant behavior by cleaving the cell-adhesion molecule E-cadherin and liberating TGF-β. They speed up tumorigenesis by inducing angiogenesis (Bergers et al., 2000), and induce tumor cells invasion and metastasis process by the degradation of surrounding ECM.
MMP-7 is involved in the progression of many cancers, for example, gastric adenocarcinoma, colon cancer, hepatocellular carcinoma, pancreatic cancer, breast cancer, cervical and ovarian cancer. Pertinent to this study, MMP7 deletion in ApcMin/+ mice has been shown to reduce strongly the intestinal tumor burden (Wilson et al., 1997). Wang et al. have shown that MMP-7 is overexpressed in epithelial ovarian cancer, and recombinant MMP-7 promotes invasion in vitro (Wang et al., 2005). These observations suggest that MMP-7 is a viable target for the treatment of ovarian cancer.
MMP19 is widely expressed in normal human tissues. However, it is associated with ovulation and angiogenic processes and deregulated in diverse pathological conditions such as rheumatoid arthritis and cancer, suggesting its importance in cancer development (Pendas et al., 2004; Chan et al., 2011). MMP19-null mice show a decreased susceptibility to skin tumors induced by chemical carcinogens. Moreover, MMP19 can be used as a marker for tumor invasiveness in patients with oropharyngeal squamous cell carcinoma. MMP19 is highly expressed in astroglial tumors, and its expression might facilitate the invasion of glioma cells through brain extracellular matrix (Lettau et al., 2010).
In this study, on the basis of the observation that MMP7 and MMP19 is overexpressed in ovarian tumor tissue, we treated ovarian cancer cells SKOV3 and A2780 with triptolide. This treatment inhibited the migration and invasion of ovarian cancer cells, decreased the expression of MMP7 and MMP19 through inhibition of their promoters, and induced E-cadherin expression.
Results
MMP7 and MMP19 expression increased in ovarian cancer tissue
To investigate the involvement of MMP7 and MMP19 in ovarian cancer, we examined MMP7 and MMP19 expression in ovarian cancer tissue and in adjacent normal tissue using Western blotting. MMP7 and MMP19 expression was elevated significantly in 46% and 38% of analyzed carcinoma tissue samples, respectively, in comparison with adjacent normal tissue controls, indicating crucial roles for these proteins in ovarian cancer development. Representative photographs are shown in Figure 1.
MMP7 and MMP19 expression increased in human ovarian cancer tissue. Equal amounts of protein lysates from ovarian cancer tissue (lanes T) and adjacent normal tissue (lanes N) from three different patients were analyzed by Western blotting. Tubulin was used as an internal control. Density of bands was quantified by Total lab software.
Triptolide inhibited ovarian cancer cells proliferation and migration
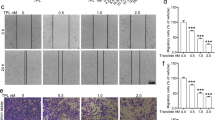
First, we evaluated the effect of triptolide on ovarian cancer cell proliferation. Figure 2A shows that triptolide inhibited ovarian cancer cell proliferation in a dose-dependent manner in both SKOV3 and A2780 cells. This effect is first clearly noticeable after 72 h incubation with 50 nM triptolide. To study the effect of triptolide on ovarian cancer cell motility, we used scratch wound healing assay, measuring the extent of cell migration into the scratched area. Control cells were confluent at 48 h after scratching. To examine the effect of triptolide on cell migration, we selected the concentration of triptolide that did not affect cells' proliferation, and the cells were incubated with 15 nM triptolide (the concentration not affecting cell proliferation). Cell migration was significantly inhibited, by 23% for SKOV3 cells and 48% for A2780 cells, 48 h after wounding (Figures 2B and 2C). Paclitaxel is a chemotherapy agent used frequently to treat ovarian cancer patients; we used it as a positive control to evaluate the anti-cancer efficacy of triptolide. We observed that although Paclitaxel inhibited ovarian cancer cell migration, the Paclitaxel-treated cells migrated significantly faster than the cells treated with 15 nM triptolide.
Effects of triptolide on the migratory abilities of ovarian cancer cells. (A) SKOV3 and A2780 cells were exposed to triptolide at the concentration from 5 to 500nM, survival of cells were detected by MTT assay. (B) After incubation with 15 nM triptolide or Paclitaxel for 24 h and 48 h, the migratory abilities of SKOV3 and A2780 cells were evaluated by the wound healing assay. Photographs shown here are representative images for SKOV3 cells. (C) Relative wound closure was calculated by comparing wound width to the wound width for the control cells. *P < 0.01 vs. untreated cells, #P < 0.01 vs. Paclitaxel.
Triptolide inhibited ovarian cancer cell invasion
We next investigated the effect of triptolide on invasive properties of ovarian cancer cells. Matrigel transmembrane invasion assay was used; the cells that migrated across a membrane toward the source of serum attractant were stained with crystal violet. Representative images of staining for migrated SKOV3 cells are shown in Figure 3A. We found that 15 nM triptolide markedly blocked the invasive capacity of SKOV3 and A2780 cells (Figure 3B). Although Paclitaxel inhibited ovarian cancer cell invasion, the inhibitive effect of triptolide was significantly greater than observed for the cells incubated with 15 nM Paclitaxel for 48 h (Figure 3C).
Effect of triptolide on the invasive abilities of ovarian cancer cells. SKOV3 and A2780 cells were treated with triptolide or Paclitaxel at 15nM. (A) Representative photos of SKOV3 cells that migrated through 8 µm pore transwell membranes with matrigel. (B, C) Invasiveness of SKOV3 and A2780 cells was evaluated by counting cells that migrated through 8 µm pore transwell membranes with matrigel. *P < 0.01 vs. untreated cells, #P < 0.01 vs. Paclitaxel.
Triptolide inhibited MMP7 and MMP19 expression in ovarian cancer cells
ECM degradation mediated by MMPs such as MMP7 and MMP19 is an essential step in tumor invasion and metastasis. Since MMP7 and MMP19 are overexpressed in ovarian cancer tissue, we investigated the effect of triptolide on MMP7 and MMP19 expression in ovarian cancer cells. SKOV3 and A2780 cells were exposed to 0, 5 or 15 nM triptolide for 24 h. The results of Western blotting showed that the levels of MMP7 and MMP19 expression were reduced in both cell lines (Figure 4). Similarly, RT-PCR showed that MMP7 and MMP19 expression decreased on the mRNA level. Furthermore, we employed luciferase reporter assay to evaluate MMP7 and MMP19 promoter activities. Figure 5 shows that in SKOV3 and A2780 cells, MMP7 and MMP19 promoter activity were substantially inhibited in a dose-dependent manner when the cells were incubated with triptolide (Figure 5). Knocking down MMP7 and MMP19 by siRNA significantly reduced cells migration (Figure 5B). We next overexpressed MMP7 and MMP19 in SKOV3 and A2780 cells by transfecting the cells with HA-tagged MMP7 and MMP19 vector (Figure 5B). We found that overexpression of MMP7 or MMP19 partially alleviates the inhibitory effect of triptolide on invasion of ovarian cancer cells.
Effect of triptolide on MMP7 and MMP19 expression. (A) SKOV3 and A2780 cells were incubated with triptolide at 0, 5 and 15 nM concentration for 24 h. MMP7 and MMP19 expression at protein level were evaluated by Western blot. Tubulin was used as an internal control. (B) SKOV3 and A2780 cells were incubated with triptolide at 0, 5 and 15 nM for 24 h. MMP7 and MMP19 expression at mRNA level were evaluated by RT-PCR. GAPDH was used as an internal control.
Effect of triptolide on MMP7 and MMP19 promoter activity. (A) MMP7 and MMP19 promoter activities were tested by luciferase assay after treatment with 0, 5 and 15 nM triptolide for 24 h. *P < 0.01 vs. untreated cells. (B) MMP7 and MMP19 were knocked down by siRNA (labeled as MMP7 KD or MMP19 KD) or overexpressed (labeled as MMP7 or MMP 19) in SKOV3 and A2780 cells. Invasiveness of SKOV3 and A2780 cells was evaluated by counting cells that migrated through 8 µm pore transwell membranes with matrigel. *P < 0.01 vs. control cells, #P < 0.01 vs. Triptolide-treated cells.
Triptolide up-regulated E-cadherin expression in ovarian cancer cells
Detachment from the neighboring cells is a crucial step for cancer cell metastasis. E-cadherin is a key molecule in the control of cell-cell adhesion; in cancer cells, this molecule is either partially or entirely missing from the membranes. We tested the effect of 0, 5 or 15 nM triptolide on E-cadherin expression. Western blotting results showed that E-cadherin expression increased in a dose-dependent manner after triptolide treatment for 24 h (Figure 6A). Using SKOV3 and A2780 cells transfected with shRNA against E-cadherin, we found that the resulting suppression of E-cadherin expression partially abolished the inhibitory effect of triptolide on invasion of ovarian cancer cells (Figure 6B).
Effect of triptolide on E-cadherin expression. (A) SKOV3 and A2780 cells were incubated with triptolide at 0, 5 and 15 nM for 24 h. E-cadherin expression at protein level was evaluated by Western blotting. Tubulin was used as an internal control. (B) E-cadherin expression was suppressed (E-cadherin KD) in SKOV3 and A2780 cells by transfecting with shRNA against E-cadherin. Invasiveness of SKOV3 and A2780 cells was evaluated by counting the cells that migrated through 8 µm pore transwell membranes with matrigel. *P < 0.01 vs. control cells, #P < 0.01 vs. Triptolide-treated cells.
Triptolide inhibited ovarian cancer tumors formation and metastasis in vivo, suppressed MMP9 and MMP17 expression, and enhanced the expression of E-cadherin
Next, we turned to the in vivo analysis of triptolide effect on ovarian cancer. When triptolide was administrated orally to mice with established tumor xenografts, it had a significant, dose-dependent inhibitory effect on the growth of tumors, beginning with the dosage of 0.1 to 1 mg/kg (Figure 7A). Furthermore, Western blots of protein extracts from the tumor xenografts showed that triptolide had the same effect in vivo as in vitro. The levels of MMP9 and MMP17 were lower, and E-cadherin levels increased in the tumors of mice treated with triptolide (Figure 7B).
Triptolide inhibited tumor formation in nude mice. (A) SKOV3 cells were injected subcutaneously into nude mice. Triptolide at 0, 0.1, 0.3 or 1 mg/kg/day was orally administered daily for three weeks, and tumor xenografts were removed and weighed. *P < 0.05 comparing to untreated mice. (B) Protein lysates were prepared from tumors. MMP7, MMP19 and E-cadherin were detected by Western blot. (C) SKOV3 cells were injected (i.p.) into nude mice. Triptolide at 0 and 1 mg/kg/day was orally administered daily for five weeks, and metastasis nodules were examined and quantified. *P < 0.05 comparing to untreated mice.
35 days after nude mice were injected with SKOV3 cells (i.p.), disseminated and enlarged metastatic nodules were found throughout the peritoneal cavity in control mice. The number of metastatic nodules was significantly reduced (by ~80%) in mice treated with triptolide at the concentration of 1 mg/kg (Figure 7C). These results suggested that triptolide can efficiently inhibit the metastasis of SKOV-3 ovarian cancer cells.
Discussion
Ovarian cancer is one of the major causes of cancer deaths in women (Bomalaski, 1999). It has a high mortality due to its clinically occult dissemination and metastasis (Saad et al., 2010). The spread of ovary cancer cells occurs by direct migration and invasion of adjacent organs, as well as throughout the peritoneal cavity by flow of peritoneal fluid (Naora and Montell, 2005; Gubbles et al., 2010). Because of this unique and furtive mode of dissemination, efforts to develop chemotherapies for ovarian cancer have been largely unsuccessful (Dutta et al., 2010; McKenzie et al., 2011). Therefore, a better understanding of the cellular and molecular mechanisms that are responsible for ovarian cancer metastasis is needed.
Tumor invasion and metastasis are associated with a complex cascade of proteolytic events, including degradation of extracellular matrix (ECM) proteins and basement membrane (Coussens et al., 2002). Matrix metalloproteinases (MMPs) are proteolytic enzymes that play an important role in cancer progression through enhancement of cell motility, invasion, and lymph node metastasis by degrading components of the basement membrane, thereby facilitating carcinoma cell intravasation and dissemination (Curran and Murray, 1999; Nagase and Woessner, 1999). MMP group of proteins expressed and secreted by carcinoma and stromal cells consists of 23 members including 17 secreted as soluble enzymes, and 6 membrane type-metalloproteinases (Radisky and Radisky, 2010). MMPs may also modulate carcinoma cells physiology in vivo through cleaving of growth factors, cell surface receptors, cell adhesion molecules, or chemokines/cytokines. For instance, as a consequence of cleaving of the pro-apoptotic factors, MMPs may be able to produce a more aggressive phenotype via generation of apoptosis-resistant cells (Gonzalez et al., 2010). In the present study, we detected increased expression of MMP7 and MMP19 in ovarian cancer tissue in comparison with the adjacent normal tissue, suggesting a crucial role for those proteins in ovarian cancer progression.
Triptolide has attracted a lot of attention because of its potential anti-inflammatory and anti-cancer activities (Yang et al., 2003; Corson and Crews, 2007). Our previous study has shown that it inhibits the proliferation of colorectal cancer cells in vitro and in vivo by interrupting the IL6R-JAK/STAT pathway (Wang et al., 2009). In this study, we examined the effect of triptolide on ovarian cancer cells. The results showed that triptolide significantly inhibited migration and invasion of ovarian cancer cells SKOV3 and A2780 in vitro. Since ECM degradation mediated by MMPs is an essential step in tumor invasion and metastasis, we hypothesized that triptolide might be able to alter the expression and activity MMPs such as MMP7 and MMP19. We observed that triptolide suppressed MMP7 and MMP19 expression at both mRNA and protein levels in SKOV3 and A2780 cells. Further investigation showed that MMP7 and MMP19 promoter activities were substantially inhibited in a dose-dependent manner by treatment with triptolide. In vivo, triptolide significantly reduced the growth of primary SKOV3 xenografts in nude mice. MMP7 and MMP19 expression in tumor tissues dramatically decreased in mice treated with triptolide, confirming the results of in vitro analysis.
The separation of cancer cells from the surrounding cells is another important step in cancer dissemination and metastasis process (Baum et al., 2011). Cells are held together by the interaction of cell-cell adhesion molecules. Cadherins, a family of intercellular adhesion protein molecules including E-cadherin, play a major part in mammalian cell adherence (Berx et al., 2001). E-cadherin mediates intercellular adhesion through homophilic associations with the extracellular domains of E-cadherin of a neighboring cell. In cancer cells, E-cadherin is down-regulated, allowing cells to detach from each other and from the ECM (Cavallaro et al., 2002). Some chemical compounds have been reported to retard cancer cell motility by increasing the expression of E-cadherin. For instance, Artemisinin enhances E-cadherin activity, resulting in greater cell-cell adhesion, and therefore reduces metastasis of hepatocellular carcinoma cells (Weifeng et al., 2011). Here, we observed that triptolide markedly increased the expression of E-cadherin not only in SKOV3 and A2780 cells in vitro, but also in xenographs and metastasis of SKOV3 in vivo, in a dose-dependent manner. This upregulation of E-cadherin expression might at least partially decrease cell migration and invasion, due to the enhanced cell-cell adhesion.
In summary, we found that triptolide significantly inhibited migration and invasion in ovarian cancer cells. This was achieved by suppression of MMP7 and MMP19 expression. We also observed that triptolide up-regulated the E-cadherin expression, thereby improving cell-cell adhesion, resulting in retarded cell motility. This study suggests that triptolide is potentially a very useful compound for the development of chemotherapeutic treatments for ovarian cancer.
Methods
Tissue sample collections
A collection of tissue specimens, 20 normal and 20 ovarian tumor samples (5 serous borderline ovarian tumors, and 15 serous ovarian cystadenocarcinomas), were obtained from the Department of Gynaecology and Obstetrics of Tangdu Hospital, with Institutional Review Board approval.
Cell culture
Human ovarian cancer cell lines SKOV3 and A2780 were cultured on cell plates at 37℃, in 5% CO2, in RPMI1640 (Sigma) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 0.1 mg/ml streptomycin.
Drug preparation
Triptolide was purchased from Sigma, and dissolved in dimethyl sulfoxide (DMSO) to a stock concentration of 500 nM. The solution was filtered through a 0.22 µm micropore filter, stored at -20℃, and diluted in cell culture medium.
MTT assay
SKOV3 and A2780 cells were plated in 96-well plates (5 × 103/well). 24 h later, triptolide was added to a final concentration of 0, 1.5, 5, 15, 50, or 150 nM for 72 h. 20 µl of 5 mg/ml MTT (Sigma, St. Louis, MO) was added to each well for 4 h at 37℃. 150 µl of dimethylsulfoxide was added to dissolve the crystals. The results were obtained using an enzyme-linked immunosorbent assay reader (Bio-RAD, San Diago, CA); the measurement wavelength was 490 nm.
Wound healing assay
Wound healing assays were used to evaluate cell migration capability in 2D space. Confluent SKOV3 and A2780 cells were treated with triptolide or Paclitaxel (15 nM) for 48 h. Then a scratch wound in the monolayer was made by dragging a 1 ml pipette tip across the layer. Cells were cultured with RPMI1640 containing triptolide or Paclitaxel as described above, and the extent of wound closure was followed by microscopy at 24 h and 48 h. The experiments were repeated three times.
Invasion assay
Invasion assays were used to investigate cell migration capability in 3D gel. Briefly, SKOV3 and A2780 cells were treated with triptolide at the concentration of 15 nM for 24 h, and then 3 × 104 cells were seeded into the upper chambers of 8 µm pore transwell. To examine the invasion through the matrigel barrier, 3 × 104 cells in 100 µl of RPMI1640 and 15 nM triptolide or Paclitaxel were added to the upper chamber of each well. The cells were allowed to migrate for 20 h; migrated cells were fixed, stained, counted from six random fields and the results averaged. The experiment was repeated three times.
Western blotting
Protein extraction and immunoblot analysis were performed on cells or tumor tissue lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 1% v/v Triton X-100, 1 mM EDTA, 1 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, 1 mM Na3VO4). Lysates were centrifuged and supernatants collected. Cell lysate aliquots (20 µg) were separated by SDS-PAGE, blotted onto nitrocellulose membranes, and incubated with primary antibody, either rabbit polyclonal anti-MMP7, anti-MMP19 (diluted 1:300; Abcam), anti-E-cadherin (diluted 1:1000; Cell Signaling), or tubulin (diluted 1:5000; Sigma Chemical Co.) overnight at 4℃. After repeated washing, membranes were incubated with horseradish-peroxidase-conjugated anti-mouse secondary antibody (1:2000; Santa Cruz Biotechnology). Bands were visualized using an enhanced chemiluminescence system (Amersham Pharmacia Biotech), quantified using TotalLab TL100 software (Nonlinear Dynamics, Newcastle upon Tyne, UK), and b-actin was used to normalize for different protein amounts. Each experiment was performed in triplicate.
RT-PCR
Total RNA was extracted from ovarian cancer cells using Trizol reagent (Invitrogen, Life Technologies, CA) following the manufacture's protocol. cDNA was synthesized from 2 µg of total RNA using Superscript reverse transcriptase (Life Technologies, CA). Primer sequences used for MMP7 detection were as follows, sense: 5'-GGATGGTAGCAGTC TAGGGATTAACT-3'; and antisense: 5'-GGAATGTCCCAT ACCCAAAGAA-3'. For MMP19 detection, sense: 5'-TGCC CACAGAACCCAGTCC-3', antisense: 5'-GGTATTCCCAC CTGATGGGGTAG-3'. The housekeeping gene glyceraldehyde phosphate dehydrogenase (GAPDH) was used for normalization (sense: 5'-TGATGACATCAAGAAGGTG GTGAAG-3'; and antisense: 5'-TCCTTGGAGGCCATGTG GGCCAT-3'). The amplification conditions were 95℃ for 1 min followed by 30 cycles of 94℃ for 30 s, 59℃ for 20 s, and 72℃ for 45 s with a 5 min 72℃ extension. PCR products were analyzed by 1.2% agarose gel electrophoresis with ethidium bromide for UV light transilluminator visualization.
Transfection of ovarian cancer cells
2 × 105 SKOV3 and A2780 cells were seeded out in 6-well plates. Mixture of 0.5 µg siRNA against MMP7 or MMP19 (Santa Cruz, sc-41554, 106229) with 5 µl transfection reagent (Santa Cruz, sc-29528) was added into cell culture medium. On the other hand, cells in each well were transfected with 2 µg HA-tagged MMP7 and MMP19 vectors, or shRNA against E-cadherin (Santa Cruz, sc-35242), using 6 µl Fugene 6 transfection reagent (Roche). Expression of MMP7, MMP19 and E-cadherin was detected by Western blotting.
Promoter activity
The promoter activities of MMP7 and MMP19 were analyzed using the dual luciferase assay (Promega, Madison, WI) using a firefly luciferase vector containing an 1898-bp stretch of the 5'-flanking region (-1898/LUC) of the MMP7 or MMP19 gene. For transient transfection, SKOV3 and A2780 cells (5 × 104 cells/well) were transfected with a total amount of 0.1 µg DNA using Lipofectamine 2000 (Invitrogen) in serum-free RPMI1640 containing 0.03 mm CaCl2. For each transfection 0.06 µg of -1898/LUC vector and 0.02 µg pRL of renilla luciferase vector (an internal control) were co-transfected with 0.005, 0.01, or 0.02 µg (5, 10, or 20% of plasmid DNA used for transfection) of either MMP7 or MMP19 vector. Transfected cells were lysed after 48 h of cultivation. Luminescence was measured using a single-sample luminometer (TD/20, Turner Design, CA) according to the manufacturer's manual (Promega). All assays were performed in quadruplicate in three separate experiments. The average of these quadruplicates was taken to obtain the relative luciferase activity, which is expressed in relative light units as defined by the ratio of firefly to renilla luciferase activity multiplied by the factor of 104. Data are presented as average ± SD and show the percentage of relative light units change against the control. The results were analyzed using Student's two-tailed paired t-test.
Tumor formation and metastasis of ovarian cancer in vivo
To establish xenografts of ovarian cancer cells in mice, SKOV3 cells were injected subcutaneously into the flanks of 5- to 6-week-old BABL/c nude mice, at 5 × 106 cells/site, using 8 mice per group. After growing for 30 days, the tumor xenografts reached approximately 100 mm3. Thereafter, triptolide at 0, 0.1, 0.3 or 1 mg/kg/day was orally administered on a daily basis. At the end of three weeks, mice were sacrificed, and tumor xenografts were removed and weighed.
To examine the effect of triptolide on ovarian cancer cell metastasis, 5 × 106 SKOV3 cells were injected intraperitoneally (i.p.) into mice. Animals were killed and the number and extent of overt metastases were then quantified, 35 days after cell injection.
Statistical analysis
Results from cell phenotype characterization were analyzed by Student's t-test, using Statistical SPSS software package (SPSS Inc, Chicago). Differences were considered statistically significant at P < 0.05.
Abbreviations
- ECM:
-
extracellular matrix
- EMT:
-
epithelial-tomesenchymal transition
References
Baum B, Georgiou M . Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling . J Cell Biol 2011 ; 192 : 907 - 917
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D . Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis . Nat Cell Biol 2000 ; 2 : 737 - 744
Berx G, Van Roy F . The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression . Breast Cancer Res 2001 ; 3 : 289 - 293
Bomalaski JJ . The treatment of recurrent ovarian carcinoma: balancing patient desires, therapeutic benefit, cost containment and quality of life . Curr Opin Obstet Gynecol 1999 ; 11 : 11 - 15
Cavallaro U, Schaffhauser B, Christofori G . Cadherins and the tumour progression: is it all in a switch ? Cancer Lett 2002 ; 176 : 123 - 128
Chan KC, Ko JM, Lung HL, Sedlacek R, Zhang ZF, Luo DZ, Feng ZB, Chen S, Chen H, Chan KW, Tsao SW, Chua DT, Zabarovsky ER, Stanbridge EJ, Lung ML . Catalytic activity of matrix metalloproteinase-19 is essential for tumor suppressor and anti-angiogenic activities in nasopharyngeal carcinoma . Int J Cancer 2011 ; 129 : 1826 - 1837
Corson TW, Crews CM . Molecular understanding and modern application of traditional medicines: triumphs and trials . Cell 2007 ; 130 : 769 - 774
Coussens LM, Fingleton B, Matrisian LM . Matrix metalloproteinase inhibitors and cancer: trials and tribulations . Science 2002 ; 295 : 2387 - 2392
Curran S, Murray GI . Matrix metalloproteinases in tumour invasion and metastasis . J Pathol 1999 ; 189 : 300 - 308
Du D, Pedersen E, Wang Z, Karlsson R, Chen Z, Wu X, Brakebusch C . Cdc42 is crucial for the maturation of primordial cell junctions in keratinocytes independent of Rac1 . Exp Cell Res 2009 ; 315 : 1480 - 1489
Dutta S, Wang FQ, Phalen A, Fishman DA . Biomarkers for ovarian cancer detection and therapy . Cancer Biol Ther 2010 ; 9 : 668 - 677
Gonzalez LO, Junquera S, del Casar JM, González L, Marín L, González-Reyes S, Andicoechea A, González-Fernández R, González JM, Pérez-Fernández R, Vizoso FJ . Immunohistochemical study of matrix metalloproteinases and their inhibitors in pure and mixed invasive and in situ ductal carcinomas of the breast . Hum Pathol 2010 ; 41 : 980 - 989
Gubbels JA, Claussen N, Kapur AK, Connor JP, Patankar MS . The detection, treatment, and biology of epithelial ovarian cancer . J Ovarian Res 2010 ; 3 : 8
Jackson B, Peyrollier K, Pedersen E, Basse A, Karlsson R, Wang Z, Lefever T, Ochsenbein AM, Schmidt G, Aktories K, Stanley A, Quondamatteo F, Ladwein M, Rottner K, van Hengel J, Brakebusch C . RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes . Mol Biol Cell 2011 ; 22 : 593 - 605
Kanbe K, Chiba J, Nakamura A . Decrease of CD68 and MMP-3 expression in synovium by treatment of adalimumab for rheumatoid arthritis . Int J Rheum Dis 2011 ; 14 : 261 - 266
Kim MJ, Lee TH, Kim SH, Choi YJ, Heo J, Kim YH . Triptolide inactivates Akt and induces caspase-dependent death in cervical cancer cells via the mitochondrial pathway . Int J Oncol 2010 ; 37 : 1177 - 1185
Lettau I, Hattermann K, Held-Feindt J, Brauer R, Sedlacek R, Mentlein R . Matrix metalloproteinase-19 is highly expressed in astroglial tumors and promotes invasion of glioma cells . J Neuropathol Exp Neurol 2010 ; 69 : 215 - 223
Li H, Takai N, Yuge A, Furukawa Y, Tsuno A, Tsukamoto Y, Kong S, Moriyama M, Narahara H . Novel target genes responsive to the anti-growth activity of triptolide in endometrial and ovarian cancer cells . Cancer Lett 2010 ; 297 : 198 - 206
Li J, Zhu W, Leng T, Shu M, Huang Y, Xu D, Qiu P, Su X, Yan G . Triptolide-induced cell cycle arrest and apoptosis in human renal cell carcinoma cells . Oncol Rep 2011 ; 25 : 979 - 987
McKenzie AJ, Campbell SL, Howe AK . Protein kinase A activity and anchoring are required for ovarian cancer cell migration and invasion . PLoS One 2011 ; 6 : e26552
Nagase H, Woessner JF . Matrix metalloproteinases . J Biol Chem 1999 ; 274 : 21491 - 21494
Naora H, Montell DJ . Ovarian cancer metastasis: integrating insights from disparate model organisms . Nat Rev Cancer 2005 ; 5 : 355 - 366
Pendás AM, Folgueras AR, Llano E, Caterina J, Frerard F, Rodríguez F, Astudillo A, Noël A, Birkedal-Hansen H, López-Otín C . Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice . Mol Cell Biol 2004 ; 24 : 5304 - 5313
Radisky ES, Radisky DC . Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer . J Mammary Gland Biol Neoplasia 2010 ; 15 : 201 - 212
Saad AF, Hu W, Sood AK . Microenvironment and pathogenesis of epithelial ovarian cancer . Horm Cancer 2010 ; 1 : 277 - 290
Sorsa T, Tjäderhane L, Salo T . Matrix metalloproteinases (MMPs) in oral diseases . Oral Dis 2004 ; 10 : 311 - 318
Tan BJ, Tan BH, Chiu GN . Effect of triptolide on focal adhesion kinase and survival in MCF-7 breast cancer cells . Oncol Rep 2011 ; 26 : 1315 - 1321
Vu TH, Werb Z . Matrix metalloproteinases: effectors of development and normal physiology . Genes Dev 2000 ; 14 : 2123 - 2133
Wang FQ, So J, Reierstad S, Fishman DA . Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase . Int J Cancer 2005 ; 114 : 19 - 31
Wang Z, Jin H, Xu R, Mei Q, Fan D . Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression . Exp Mol Med 2009 ; 41 : 717 - 727
Wang Z, Pedersen E, Basse A, Lefever T, Peyrollier K, Kapoor S, Mei Q, Karlsson R, Chrostek-Grashoff A, Brakebusch C . Rac1 is crucial for Ras-dependent skin tumor formation by controlling Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo . Oncogene 2010 ; 29 : 3362 - 3373
Weifeng T, Feng S, Xiangji L, Changqing S, Zhiquan Q, Huazhong Z, Peining Y, Yong Y, Mengchao W, Xiaoqing J, Wan-Yee L . Artemisinin inhibits in vitro and in vivo invasion and metastasis of human hepatocellular carcinoma cells . Phytomedicine 2011 ; 18 : 158 - 162
Westfall SD, Nilsson EE, Skinner MK . Role of triptolide as an adjunct chemotherapy for ovarian cancer . Chemotherapy 2008 ; 54 : 67 - 76
Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM . Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin . Proc Natl Acad Sci USA 1997 ; 94 : 1402 - 1407
Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei XF, Yang J, Underhill CB, Zhang L . Triptolide inhibits the growth and metastasis of solid tumors . Mol Cancer Ther 2003 ; 2 : 65 - 72
Zhou ZL, Luo ZG, Yu B, Jiang Y, Chen Y, Feng JM, Dai M, Tong LJ, Li Z, Li YC, Ding J, Miao ZH . Increased accumulation of hypoxia-inducible factor-1α with reduced transcriptional activity mediates the antitumor effect of triptolide . Mol Cancer 2010 ; 9 : 268
Acknowledgements
This work has been supported by National Natural Science Foundation of China (Grant No.31101060).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhao, H., Yang, Z., Wang, X. et al. Triptolide inhibits ovarian cancer cell invasion by repression of matrix metalloproteinase 7 and 19 and upregulation of E-cadherin. Exp Mol Med 44, 633–641 (2012). https://doi.org/10.3858/emm.2012.44.11.072
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2012.44.11.072
Keywords
This article is cited by
-
Triptolide suppresses the growth and metastasis of non-small cell lung cancer by inhibiting β-catenin-mediated epithelial–mesenchymal transition
Acta Pharmacologica Sinica (2021)
-
Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine
Chinese Medicine (2019)
-
Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells
Biological Research (2019)
-
EMT-related protein expression in polyploid giant cancer cells and their daughter cells with different passages after triptolide treatment
Medical Oncology (2019)
-
Triptolide represses oral cancer cell proliferation, invasion, migration, and angiogenesis in co-inoculation with U937 cells
Clinical Oral Investigations (2017)