Abstract
X-linked intellectual disability (XLID) is a genetically heterogeneous disorder with more than 100 genes known to date. Most genes are responsible for a small proportion of patients only, which has hitherto hampered the systematic screening of large patient cohorts. We performed targeted enrichment and next-generation sequencing of 107 XLID genes in a cohort of 150 male patients. Hundred patients had sporadic intellectual disability, and 50 patients had a family history suggestive of XLID. We also analysed a sporadic female patient with severe ID and epilepsy because she had strongly skewed X-inactivation. Target enrichment and high parallel sequencing allowed a diagnostic coverage of >10 reads for ~96% of all coding bases of the XLID genes at a mean coverage of 124 reads. We found 18 pathogenic variants in 13 XLID genes (AP1S2, ATRX, CUL4B, DLG3, IQSEC2, KDM5C, MED12, OPHN1, SLC9A6, SMC1A, UBE2A, UPF3B and ZDHHC9) among the 150 male patients. Thirteen pathogenic variants were present in the group of 50 familial patients (26%), and 5 pathogenic variants among the 100 sporadic patients (5%). Systematic gene dosage analysis for low coverage exons detected one pathogenic hemizygous deletion. An IQSEC2 nonsense variant was detected in the female ID patient, providing further evidence for a role of this gene in encephalopathy in females. Skewed X-inactivation was more frequently observed in mothers with pathogenic variants compared with those without known X-linked defects. The mutation rate in the cohort of sporadic patients corroborates previous estimates of 5–10% for X-chromosomal defects in male ID patients.
Similar content being viewed by others
Introduction
Intellectual disability (ID) is a childhood-onset neurodevelopmental disorder commonly defined by an intelligence quotient below 70.1 It has an estimated incidence of 1–3% in Western societies.2 Causes of ID include environmental factors and genetic defects. Mutations in X-chromosomal genes are estimated to account for 5–10% of male ID patients.3 In female carriers of defects in X-chromosomal genes, inactivation of the X-chromosomes can be skewed (ie, non-random), which may be used as a predictive marker for such mutations.4 X-linked intellectual disability (XLID) is characterized by extensive genetic heterogeneity; to date, mutations in more than 100 genes on the X-chromosome are known to be associated with XLID.5 With the exception of Fragile X syndrome that is at cause in about 25% of XLID patients, the other XLID genes individually only account for a small percentage of patients.6 This has rendered routine testing of these genes impractical in patients with nonspecific clinical features. Next-generation sequencing (NGS) technologies have recently provided the means for overcoming this problem by cost-effective parallel analysis of large numbers of genes. In contrast to the use of NGS as a research tool for which reduced sensitivity can be acceptable, application of NGS in diagnostics demands for more rigid quality standards, as insufficient coverage and/or inaccurate bioinformatic analysis will lower the diagnostic yield. In order to maximize sensitivity and specificity, targeted gene panels make use of high coverage sequencing at relatively low costs.7
Here, we report on the screening of 107 XLID genes that were analysed by targeted enrichment and subsequent NGS in a cohort of 150 male ID patients.
Patients and methods
Patients
The study comprised 150 male ID patients in whom the cause of the disease was not known. One hundred patients were sporadic cases, that is, they had no other family members with cognitive defects. Fifty patients had a family history suggestive of XLID, that is, they had either affected brothers or male maternal relatives or less severely affected female family members compatible with an X-linked mode of inheritance.
Chromosome aberrations were ruled out by microarray analysis (array CGH or SNP array) in all patients before they were included into this study. Fragile X syndrome was excluded in all patients by testing for CGG repeat expansions in the 5′ untranslated region of FMR1. Patients with a history of seizures were also tested for the recurrent 24 bp duplication in exon 2 of ARX.
All patients were seen by a clinical geneticist to rule out obvious syndromic disorders. Four patients were excluded in this way from the main cohort because recognizable syndromes (Allan-Herndon-Dudley syndrome, Börjeson-Forssman-Lehmann syndrome and Christianson syndrome) were suspected and subsequently confirmed by conventional Sanger sequencing of the respective genes. We also excluded patients with indications for autosomal dominant disorders (eg, if they had a similarly affected father) or autosomal recessive defects (eg, if they had similarly affected sisters and consanguineous parents).
In addition to the 150 male ID patients of the main cohort, we also analysed a female sporadic patient with severe ID and epilepsy because she had strongly skewed X-inactivation (patient 19, Table 1 and Supplement).
Next-generation sequencing
DNA samples from peripheral blood cells were prepared according to standard protocols. Fragmentation of patient DNA samples was carried out with a Covaris S2 ultrasonicator, generating fragments of 200–500 bp in size (Covaris, Woburn, MA, USA). For library generation of the fragmented samples, the Illumina Paired-End DNA Sample Preparation Kit was used (Illumina, San Diego, CA, USA).
Enrichment of the target sequences, which included nearly all coding and flanking intronic regions of the X-chromosome, was performed using the Agilent SureSelectXT X-Chromosome in-solution target enrichment kit (Agilent, Santa Clara, CA, USA) followed by individual barcoding of each sample.
Sequencing was performed using the Illumina GAIIx sequencer (2 × 76 paired-end sequencing). For each lane of a flow cell, six patient samples were pooled.
The 76 bp reads were preprocessed with adapter trimming (cutadapt)8 duplicate removal (picard-tools version 1.75; http://picard.sourceforge.net). The reads were subsequently mapped to the human reference genome (hg19) using BWA (version 0.5.9) and Stampy (version 1.0.17).9, 10 Variants were detected using SAMtools (version 0.1.18) and annotated with data from the 1000 Genomes Project, dbSNP and the NHLBI GO Exome Sequencing Project using ANNOVAR (version 2012-05-25).11, 12
For gene dosage analysis (hemizygous deletions in males), a simple but effective statistical approach was used. First, the normalized average read depth of each exon was calculated (ie, the average read depth of an exon divided by the average read depth of all exons of the sample). Then, the mean and standard deviation (SD) of the normalized average read depth over all samples were calculated for each exon. All normalized read depth values outside a three SD window around the mean were PCR-validated after apparent false positives had been excluded by inspection in the Integrative Genomics Viewer (IGV; http://www.broadinstitute.org/igv/).
Analysis of variants was restricted to the coding and flanking intronic sequences of the following 107 XLID genes: ACSL4, AFF2, AGTR2, AIFM1, AP1S2, ARHGEF6, ARHGEF9, ARX, ATP6AP2, ATP7A, ATRX, BCOR, BRWD3, CASK, CDKL5, CLCN4, CUL4B, DCX, DKC1, DLG3, DMD, EIF2S3, FANCB, FGD1, FLNA, FMR1, FTSJ1, GDI1, GK, GPC3, GRIA3, HCCS, HCFC1, HDAC8, HPRT, HSD17B10, HUWE1, IDS, IGBP1, IKBK, IL1RAPL1, IQSEC2, KDM5C, KDM6A, KIAA2022, KLF8, L1CAM, LAMP2, LAS1L, MAGT1, MAOA, MBTPS2, MECP2, MED12, MID1, MTM1, NAA10, NDP, NDUFA1, NHS, NLGN3, NLGN4X, NSDHL, NXF5, OCRL, OFD1, OPHN1, OTC, PAK3, PCDH19, PDHA1, PGK1, PHF6, PHF8, PLP1, PORCN, PQBP1, PRPS1, PTCHD1, RAB39B, RAB40AL, RBM10, RPL10, RPS6KA3, SHROOM4, SLC6A8, SLC9A6, SLC16A2, SMC1A, SMS, SOX3, SRPX2, SYP, SYN1, THOC2, TIMM8A, TSPAN7, UBE2A, UPF3B, WDR45, ZDHHC9, ZDHHC15, ZMYM3, ZNF41, ZNF81, ZNF674 and ZNF711. References for all genes are in Supplementary Table S1 of the review by Lubs et al,3 except for SYP, ZNF711, AIFM1, ZMYM3, KDM6A and WDR45.13, 14, 15, 16, 17, 18
Variant classification
All coding variants and variants in the exon-intron boundaries (+/−10 bp) were classified following the suggestions of Plon et al.19 Only probably (VUS4) or definitely pathogenic variants (VUS5) were used to establish a genetic diagnosis. Novel missense variants, synonymous variants with potential effects on splicing and in-frame deletions or insertions were rated VUS3 (uncertain significance) regardless of in silico predictions. A novel missense variant was assigned as probably pathogenic (VUS4) if segregation was informative and the clinical phenotype met published expectations. Nonsense, frameshift and splicing variants (+/− 2 bp) were also classified as VUS4 if they were novel.
Sanger sequencing
Putatively pathogenic variants were validated by conventional Sanger sequencing. This was followed by segregation analysis of confirmed variants in affected and unaffected members of the respective families.
X-inactivation analysis
A total of 107 maternal DNA samples were available for X-inactivation analyses. We used the polymorphic CAGn repeat within the human AR gene to assess the relative methylation status of both chromosomes after methylation-sensitive restriction enzyme digest.20 X-inactivation was considered as skewed (non-random) if the ratio of the two alleles exceeded 80:20.
Results
NGS sequencing characteristics
On average, 9.26 million reads of 76 bp length were generated per patient sample. A minimum 10-fold coverage per base was on average achieved for 94.61% of the target region. Regions with insufficient coverage were mostly recurrent and usually characterized by a high GC content. Specifically, parts of the coding regions of IKBGK, IQSEC2, ARX, MBTBS2, PCDH19, SLC16A2, SOX3 and ZNF81 could not be analysed reliably. ABCD1 was altogether excluded from this study because sequence homology to other parts of the genome hampered analysis by this approach. Repetitive DNA structures such as trinucleotide DNA repeats could not be analysed with this method. In particular, this concerned the CGG-trinucleotide stretch in the 5′ UTR of FMR1, expansion of which had been excluded in all patients before they were subjected to analysis by this panel. In patients with epilepsy, the recurrent 24 bp duplication in exon 2 of ARX was excluded by separate tests. The data were also analysed for sequencing drop-outs of entire exons which would suggest larger deletions. In one patient (patient 16, Table 1 and Supplement), a deletion of a single exon (exon 1 of SLC9A6) was detected in this way and confirmed by PCR. In contrast, the presence of larger duplications could not be excluded. Duplications larger than 100 kb would, however, have been detected by the array-based chromosome tests that had been performed in all patients before they were included in this study.
Variants with a minor allele frequency of more than 1%, intronic variants with a distance of more than 10 nucleotides to the nearest exon and synonymous variants without a predicted splicing effect were excluded from further analysis.
Pathogenic variants
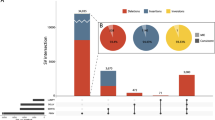
In total, 18 probably or definitely pathogenic variants in 13 XLID genes were detected in the 150 patients of the main cohort (Table 1 and Supplement).
In the group of 50 patients with a family history suggestive of XLID, 13 pathogenic variants (26%) were detected in the following genes: AP1S2 (family 1), ATRX (families 2, 3 and 4), CUL4B (families 5 and 6), IQSEC2 (family 7), KDM5C (families 8 and 9), MED12 (family 10), OPHN1 (family 11), UPF3B (family 12) and ZDHHC9 (family 13).
Among the 100 sporadic patients, five pathogenic variants (5%) were detected in CUL4B (patient 14), DLG3 (patient 15), SLC9A6 (patient 16), SMC1A (patient 17) and UBE2A (patient 18).
In the female ID patient with skewed X-inactivation (patient 19), a nonsense variant was detected in IQSEC2 [NM_001111125.2: c.3163C>T; p.(Arg1055*)] (Table 1 and Supplement).
Sanger sequencing in the male patients with a clinically diagnosed syndrome revealed a pathogenic variant in SLC16A2 [NM_006517.4: c.590G>A; p.(Arg197His)]; according to the previous version of the reference sequence (NM_006517.3), this was c.812G>A; p.(Arg271His)] in patient 20 and a pathogenic variant in PHF6 [NM_032458.2: c.687 T>A; p.(His229Gln)] in patient 21 (Table 1 and Supplement).
The variants listed in Table 1 were submitted to the Leiden Open Variation Databases (www.lovd.nl).
Variants of uncertain significance
We also identified 42 rare variants (missense, synonymous or in-frame deletions) which did not qualify as benign polymorphisms (Supplementary Table 1). A pathogenic role for these variants was uncertain because there were either no family members available for segregation analyses, or the clinical features did not fit those that would have been expected for the respective gene, or the in silico prediction scores argued against a severe disruption of the gene product. With the availability of larger databases and/or functional studies, we expect that many (or most) of these variants will turn out to be benign, but some variants might eventually reveal themselves as causative defects.
X-inactivation in mothers
X-inactivation analysis was informative in mothers of 95 families, that is, two alleles of different size were present. Skewing was detected in 23 out of 95 mothers (24.2%); details of the ratios of skewed versus random X-inactivation in subgroups (families with pathogenic variants or VUS3 variants, families with sporadic patients or multiple affected members) are listed in Table 2. In families with pathogenic variants, skewing was more frequent (five of eight families, 62.5%) compared with the general cohort (24.2%), but a considerable part of this group (three out of eight families, 37.5%) had random X-inactivation (Table 2). Some female family members with skewed X-inactivation were also mildly retarded (eg, in family 9 with a pathogenic variant in KDM5C).
Discussion
In this study, we have analysed a panel of 107 XLID genes by NGS. Outstanding sequence coverage was achieved with approximately 95% of all coding bases covered at >10 reads. As males are hemizygotes for X-linked genotypes, this coverage should have been sufficient to detect >99% of all covered SNVs.
In a cohort of 150 male ID patients, we have identified 18 pathogenic variants in these 107 genes. As expected, the diagnostic yield was higher among the 50 patients with a family history suggestive of X-chromosomal inheritance (26%) compared with the 100 sporadic patients (5%).
Bearing in mind that our cohort did not represent an unbiased sample of ID patients, these results nevertheless allow us to draw conclusions on the collective contribution of X-chromosomal genes to ID, and on the prevalence of mutations in individual XLID genes.
Fragile X syndrome, the most frequent single cause of XLID that is estimated to account for 25%, and submicroscopic copy number variations, which have been reported to be at cause in up to 10% of XLID patients, had been excluded in all patients before they were subjected to NGS.6, 21 In patients with recognizable syndromes, the respective genes were directly tested by Sanger sequencing. This was the case in patient 20 with Allan-Herndon-Dudley syndrome, in patient 21 with Börjeson-Forssman-Lehmann syndrome (Table 1 and Supplement), and in two families with Christianson syndrome.22 Recognition of a syndrome does, however, rely on the clarity of the phenotypic manifestation and on the experience of the clinical geneticist. To avoid duplicate analyses, we refrained from direct gene testing unless the patients had unambiguous indications for a specific syndrome.
If one takes into consideration that (i) a proportion of male ID patients had been filtered out before by the criteria discussed above, (ii) mutations outside the immediate vicinity of the coding regions could not be detected, (iii) only approximately 95% of the target regions could be reliably analysed, (iv) some of the variants of uncertain significance may in fact be pathogenic, and (v) that there may exist as yet unidentified additional XLID genes, the detection of five mutations in 100 sporadic patients is in line with previous estimates that mutations in X-chromosomal genes account for 5–10% of ID in males.3
With the exception of ATRX (pathogenic variants in three families), CUL4B (three pathogenic variants) and KDM5C (two pathogenic variants), only a single pathogenic variant was identified in each of the other 10 genes (AP1S2, IQSEC2, MED12, OPHN1, UPF3B, ZDHHC9, DLG3, SLC9A6, SMC1A, UBE2A) (Table 1). Six of those genes (AP1S2, IQSEC2, MED12, UPF3B, ZDHHC9, DLG3) have rarely been associated with ID, that is, fewer than 10 pathogenic variants have so far been reported for each individual gene.
The XLID-causing role of several of the 107 genes, which we analysed in this study, had been challenged in a recent re-evaluation based on data from large-scale exome sequencing projects.5 In none of the 17 genes that were put into question in this study and which were also included in our analysis (ATP6AP2, ZNF674, ZNF41, ZNF81, MAGT1, SRPX2, NXF5, AGTR2, MAOA, SHROOM4, KLF8, IGBP1, NLGN3, ZMYM3, KIAA2022, ZDHC15, NAA10), we detected pathogenic variants in our cohort of 150 ID patients.
Apart from broadening the mutational spectrum of rare XLID genes, the patients reported here also expand the clinical features associated with the respective genes. For example, gall bladder agenesis (as in the two brothers of family 4) is possibly an under-appreciated feature in patients with ATRX mutations. In patients with CUL4B mutations, neither cleft palate nor optic atrophy (as in family 5) nor microcephaly (as in patient 14) has been reported so far. Microcephaly is also an unusual feature in patients with MED12 mutations (family 10).
XLID is not necessarily restricted to male mutation carriers. Among the families of the present study, this is illustrated by the affected women in family 9 (KDM5C mutation) who remind us of taking disease expression in females into consideration for genetic counselling.
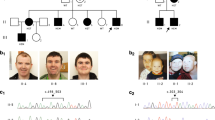
The interpretation of intronic variants is a particular challenge. We have encountered this problem in family 2 with an intronic base pair exchange (c.6975+5G>A) in ATRX. Its pathogenic nature could only be established after segregation analyses and RT-PCR studies.
Egg donation is legally restricted in many countries, albeit not for genetic reasons. Patient 16, who suffered from Christianson syndrome, is an example for the genetic risks that can be associated with egg donation if the donor carries a pathogenic variant in an X-chromosomal gene.
We had also tested a sporadic female patient in whom strongly skewed X-inactivation hinted at an X-chromosomal disorder (patient 19, Table 1 and Supplement). This 16-year-old patient had severe ID, epilepsy and borderline macrocephaly. Her parents were healthy, and she had no other family members with ID or epilepsy. Previous genetic tests revealed normal results (array-CGH, FraX, MECP2, CDKL5). A stop mutation was detected in IQSEC2 (Table 1). IQSEC2 mutations had originally been identified in male ID patients (as in family 7 in the present report), and carrier females in the respective families were healthy.23, 24 Only recently, a de novo stop mutation in IQSEC2 was reported in a 3-year-old girl with epileptic encephalopathy.25 The severity of the clinical problems of the female patient reported here is similar to that of the male patients with truncating IQSEC2 mutations and may be explicable by skewing in favour of the X-chromosome with the mutated allele. However, even if this assumption is true, the cause of such disadvantageous skewing is presently not clear. A defect in a crucial gene on the other X-chromosome might provide a possible explanation. The female patient presented here, together with the girl that was reported by the Epi4K Consortium25 extend the clinical manifestations of IQSEC2 mutations beyond the male sex and should be considered in females with otherwise unexplained ID and epilepsy.
X-inactivation analyses in mothers of patients of our cohort have confirmed previous observations that skewing can be an indicator for an X-linked defect, but random X-inactivation (which was found in three out of eight informative carrier mothers, or 37.5%, of patients with pathogenic variants in this study) does not preclude an X-linked disorder. In routine diagnostics, X-inactivation studies can thus be helpful in directing the order of genetic tests, but they should be applied with caution.
The XLID genes KDM5C and SMC1A had been reported to escape X-inactivation.26, 27 As in the girl with the IQSEC2 mutation (patient 19) mentioned above, the mechanisms that caused skewing in female members of families 8 and 9 (with KDMC5 mutations) and in family 17 (with mutation in SMC1A) await their elucidation.
In conclusion, targeted NGS-based analysis is a powerful tool to detect disease-causing mutations in XLID patients. The results of this study broaden the mutational spectrum of rare XLID genes, and they provide insight into the relative contribution of individual genes in this highly heterogeneous disorder. The application of this approach to large patient cohorts in routine diagnostics will provide the basis for a comprehensive and detailed picture of the genetic landscape of XLID.
References
Ellison J, Rosenfeld J, Shaffer L : Genetic basis of intellectual disability. Annu Rev Med 2013; 64: 441–450.
Leonard H, Wen X : The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev 2002; 8: 117–134.
Lubs H, Stevenson R, Schwartz C : Fragile X and X-linked intellectual disability: four decades of discovery. Am J Hum Genet 2012; 90: 579–590.
Plenge R, Stevenson R, Lubs H, Schwartz C, Willard H : Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. Am J Hum Genet 2002; 71: 168–173.
Piton A, Redin C, Mandel J : XLID-causing mutations and associated genes challenged in light of data from large-scale human exome sequencing. Am J Hum Genet 2013; 93: 368–383.
Ropers H : Genetics of early onset cognitive impairment. Annu Rev Genomics Hum Genet 2010; 11: 161–187.
Rehm H : Disease-targeted sequencing: a cornerstone in the clinic. Nat Rev Genet 2013; 14: 295–300.
Martin M : Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal 2011; 17: 10–12.
Li H, Durbin R : Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010; 26: 589–595.
Lunter G, Goodson M : Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res 2011; 21: 936–939.
Li H : A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011; 27: 2987–2993.
Wang K, Li M, Hakonarson H : ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010; 38: e164.
Saitsu H, Nishimura T, Muramatsu K et al: De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet 2013; 45: 445–449.
Haack T, Hogarth P, Kruer M et al: Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. Am J Hum Genet 2012; 91: 1144–1149.
Tarpey P, Smith R, Pleasance E et al: A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet 2009; 41: 535–543.
Rinaldi C, Grunseich C, Sevrioukova I et al: Cowchock syndrome is associated with a mutation in apoptosis-inducing factor. Am J Hum Genet 2012; 91: 1095–1102.
van der Maarel S, Scholten I, Huber I et al: Cloning and characterization of DXS6673E, a candidate gene for X-linked mental retardation in Xq13.1. Hum Mol Genet 1996; 5: 887–897.
Lederer D, Grisart B, Digilio M et al: Deletion of KDM6A, a histone demethylase interacting with MLL2, in three patients with Kabuki syndrome. Am J Hum Genet 2012; 90: 119–124.
Plon S, Eccles D, Easton D et al: Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat 2008; 29: 1282–1291.
Allen R, Zoghbi H, Moseley A, Rosenblatt H, Belmont J : Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 1992; 51: 1229–1239.
Whibley A, Plagnol V, Tarpey P et al: Fine-scale survey of X chromosome copy number variants and indels underlying intellectual disability. Am J Hum Genet 2010; 87: 173–188.
Riess A, Rossier E, Krüger R et al: Novel SLC9A6 mutations in two families with Christianson syndrome. Clin Genet 2013; 83: 596–597.
Shoubridge C, Tarpey P, Abidi F et al: Mutations in the guanine nucleotide exchange factor gene IQSEC2 cause nonsyndromic intellectual disability. Nat Genet 2010; 42: 486–488.
Tran Mau-Them F, Willems M, Albrecht B et al: Expanding the phenotype of IQSEC2 mutations: truncating mutations in severe intellectual disability. Eur J Hum Genet 2014; 22: 289–292.
Epi4K C, Allen A, Berkovic S et al: De novo mutations in epileptic encephalopathies. Nature 2013; 501: 217–221.
Brown CJ, Miller AP, Carrel L, Rupert JL, Davies KE, Willard HF : The DXS423E gene in Xp11.21 escapes X chromosome inactivation. Hum Mol Genet 1995; 4: 251–255.
Li N, Carrel L : Escape from X chromosome inactivation is an intrinsic property of the Jarid1c locus. Proc Natl Acad Sci USA 2008; 105: 17055–17060.
Acknowledgements
We thank the patients and their parents for their support. We also thank Juliane Gohlke for excellent technical assistance. This work was supported in part by EU funding (TECHGENE, FP7-Health 2007, B223143) and the German Intellectual Disability Network through a grant from the German Ministry of Education and Research (01GS08160).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Tzschach, A., Grasshoff, U., Beck-Woedl, S. et al. Next-generation sequencing in X-linked intellectual disability. Eur J Hum Genet 23, 1513–1518 (2015). https://doi.org/10.1038/ejhg.2015.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2015.5
This article is cited by
-
Identification of a DLG3 stop mutation in the MRX20 family
European Journal of Human Genetics (2024)
-
Whole exome sequencing revealed variants in four genes underlying X-linked intellectual disability in four Iranian families: novel deleterious variants and clinical features with the review of literature
BMC Medical Genomics (2023)
-
Structural and functional implications of SLC13A3 and SLC9A6 mutations: an in silico approach to understanding intellectual disability
BMC Neurology (2023)
-
Neurodevelopmental and psychiatric disorders in females with Turner syndrome: a population-based study
Journal of Neurodevelopmental Disorders (2021)
-
Intellectual disability genomics: current state, pitfalls and future challenges
BMC Genomics (2021)