Abstract
Nuclear import receptors of the KPNA family recognize the nuclear localization signal in proteins and together with importin-β mediate translocation into the nucleus. Accordingly, KPNA family members have a highly conserved architecture with domains that contact the nuclear localization signal and bind to importin-β. Here, we describe autosomal recessive mutations in KPNA7 found by whole exome sequencing in a sibling pair with severe developmental disability, infantile spasms, subsequent intractable epilepsy consistent with Lennox–Gastaut syndrome, partial agenesis of the corpus callosum, and cerebellar vermis hypoplasia. The mutations mapped to exon 7 in KPNA7 result in two amino-acid substitutions, Pro339Ala and Glu344Gln. On the basis of the crystal structure of the paralog KPNA2 bound to a bipartite nuclear localization signal from the retinoblastoma protein, the amino-acid substitutions in the affected subjects were predicted to occur within the seventh armadillo repeat that forms one of the two nuclear localization signal-binding sites in KPNA family members. Glu344 is conserved in all seven KPNA proteins, and we found that the Glu354Gln mutation in KPNA2 is sufficient to reduce binding to the retinoblastoma nuclear localization signal to approximately one-half that of wild-type protein. Our data show that compound heterozygous mutations in KPNA7 are associated with a human neurodevelopmental disease, and provide the first example of a human disease associated with mutation of a nuclear transport receptor.
Similar content being viewed by others
Introduction
Protein transport from the cytoplasm to the nucleus is mediated by nuclear import receptors, which recognize the nuclear localization signal (NLS) within a cargo destined for import.1, 2 Nuclear import receptors of the KPNA family, encoded by seven genes in humans, make direct contact with the NLS. KPNA proteins also contain an N-terminal domain that binds to importin-β, which mediates transient interactions with the nuclear pore complex (NPC) during the translocation step of protein import.3 As the NPC maintains a barrier that excludes diffusion-based entry of proteins >40 kDa, NLS- and receptor-dependent import is the major pathway for nuclear import in all eukaryotes. Although the basic protein architecture, including the two NLS-binding sites, is highly conserved among KPNA family members, certain KPNAs bind a wide range of NLS cargoes, whereas others appear more selective.4 Regulation of expression of particular KPNA family members and isoforms, which mediate import of specific transcription factors, appears to be important in several developmental programs,5 including neuronal differentiation of mouse embryonic stem cells.6
Several childhood neurodevelopmental disorders arise from loss-of-function mutations in transcription factors critical for early brain patterning, among them ARX-related disorders,7 TCF4-related Pitt–Hopkins syndrome,8 ZEB2-related Mowat–Wilson syndrome,9 MEF2C-related disorders,10 and FOXG1-related disorders.11 These syndromes share in common early-onset epilepsy and profound intellectual disability with abnormalities in multiple domains of behavior and neurologic function. In ARX- and FOXG1-related disorders as well as Mowat–Wilson syndrome there are abnormalities of the corpus callosum.12, 13, 14
Infantile spasms typically present in the first year of life and form an age-dependent electroclinical syndrome defined as epileptic spasms coupled with ictal electrodecrement and interictal hypsarrhythmia on electroencephalogram (EEG).15 Multiple genetic causes have been described and classified into biological subtypes16 that include mutations in genes important for neuronal migration (ie PAFAH1B1/LIS1 and TUBA1A), synaptic development and function (ie CDKL5, SPTAN1, and STXBP1), and neuronal metabolism and signaling (ie GLDC and PLCB1). An additional, biological subgroup of infantile spasms is caused by mutations in transcription factors with important roles in brain development (ie ARX, duplications of FOXG1, and deletions of MEF2C). Multiple genes have been associated with infantile spasms, but given the complexity of the disease, it seems likely that additional genetic associations remain to be identified.
Here, we report the first known family with mutations in a nuclear import receptor. Whole exome sequencing (WES) in a pedigree with two female siblings affected with infantile spasms, subsequent intractable epilepsy consistent with Lennox–Gastaut syndrome, partial agenesis of the corpus callosum, cerebellar vermis hypoplasia, and severe developmental outcome revealed compound heterozygous mutations in KPNA7. These mutations result in two amino-acid substitutions within the seventh armadillo repeat (ARM), which in KPNA family members is proximal to an NLS-binding site.2, 17 Using the paralog KPNA2 as a model, we show that the analogous Glu354Gln substitution in the affected individuals is sufficient to reduce binding to the retinoblastoma (Rb) NLS. On the basis of these data, we suggest that reduced function of a specific import receptor could define a novel biological mechanism for a neurodevelopmental disorder.
Methods
Patient ascertainment
Subjects were ascertained through the Infantile Spasms Registry and Genetic Studies (ISSRGS; approved by the IRBs of Washington University and the University of Rochester Medical Center) and the Molecular Genetic Studies of Developmental Disorders (approved by the IRB of Seattle Children’s Hospital). Retrospective clinical records, EEG tracings, and brain MRI scans were reviewed. DNA was extracted from peripheral blood using the Gentra PureGene DNA isolation kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions.
Whole exome sequencing
We performed WES of peripheral blood DNA from subject IS10-017a1. The Agilent SureSelect 50 Mb whole exome capture kit was used, and the sequence was generated on an Illumina HiSeq platform (Illumina, San Diego, CA, USA). Sequence was aligned to hg19 using BWA, and single-nucleotide variants and indels were called using GATK 1.3. Depth of coverage of the targeted exome was calculated with the GATK DepthOfCoverage walker. Annotation of variants was performed with SeattleSeq Annotation 134. Common variants were identified by filtering against the NHLBI Exome Variant Server and potential autosomal recessive variants were identified using Perl scripts publicly available on GitHub. Known variants in genes associated with infantile epilepsies and other neurodevelopmental disorders, specifically those with autosomal recessive inheritance, were queried by screening against the gene set available online in the Developmental Brain Disorders Database (DBDB 1.2). See web resources for all tools used. The WES data from IS10-017a1 is in the process of submission to dbGAP.
Sanger sequencing
Potential autosomal recessive variants found by WES were confirmed with Sanger sequencing using standard methods. We also sequenced the coding exons and exon/intron boundaries of KPNA7 in 56 subjects with genotype unknown ‘cryptogenic’ infantile spasms and 16 subjects with cerebellar vermis hypoplasia and a variety of forms of epilepsy (KPNA7 primer sequences in Supplementary Methods). Sequences were compared with normal control samples and the reference sequences for KPNA7 (NCBI reference number NM_001145715.1).
Modeling and protein analysis
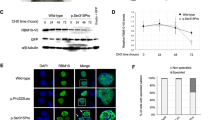
KPNA7 was threaded onto the known crystal structure of mouse KPNA2 (PDB 1PJM)17 using SPDBV and SwissModel.18, 19 Images of KPNA2 and threaded KPNA7 were generated using PyMol (The PyMol Molecular Graphics System, Version 1.5.0.4; Schrödinger, LLC, New York, NY, USA). As KPNA7 binds weakly to the Rb NLS, we used the closest family member, KPNA2, to assess the effect of the amino-acid substitution Glu354Gln on NLS-binding activity.20 Mutations were introduced into the T7-based KPNA2 plasmid by Quik-Change Mutagenesis (Promega, Madison, WI, USA). Binding assays were performed as described previously.20 In brief, a glutathione S-transferase fusion of the C terminus of Rb (amino acids 511–642) that includes the NLS was immobilized on glutathione beads and combined with 35S-labeled KPNA2 (WT and Glu354Gln forms) generated by in vitro translation using the TNT Coupled Reticulocyte Lysate System (Promega). Following SDS-PAGE and autoradiography, the films were quantified by densitometry and the bound fractions were normalized to the input.20
Results
Clinical phenotype
Subject IS10-017a1 was 8 years old at the time of study (Figure 1a and b), and was born to non-consanguineous parents with healthy male offspring. She presented with seizures on the first day of life consisting of whole body stiffening and eye rolling. She was started on phenobarbital and an interictal EEG at the time was normal. At 3 months she developed infantile spasms and multiple EEG studies demonstrated features of hypsarrhythmia, including high voltage, multifocal epileptiform discharges, and electrodecremental responses time-locked to epileptic spasms (Figure 2a). She subsequently had intractable epilepsy with EEG features consistent with Lennox–Gastaut syndrome (Figure 2b). The details of her seizure semiology, epilepsy course, and response to treatment are found in Supplementary Data.
Representative EEG tracings of subjects with KPNA7 mutations showing evolution from infantile spasms to Lennox–Gastaut syndrome. (a) Subject IS10-017a1 at age 7 months showing ictal electrodecrement with background delta activity. (b) At age 24 months she had multifocal epileptiform discharges with high amplitude slowing. (c) Subject IS10-017a2 at 4 months with ictal electrodecrement time-locked with an epileptic spasm. (d) At 4 years and 11 months, her EEG showed slow spike and wave discharges followed by a period of abrupt attenuation with superimposed fast frequency activity consistent with tonic seizures. Measurement bar: vertical axis=100 μV; horizontal axis=1 s.
Developmentally at age 8 years she was able to sit independently and roll over. Visual fixation and tracking was poor. She had no verbal language, made screaming vocalizations and head shaking. Physical exam was notable for weight at 4th percentile for age, head circumference at the 50th percentile, mild hypertelorism, and scoliosis. Brain MRI revealed thinning of the splenium of the corpus callosum, cerebellar vermis hypoplasia with posterior fossa cysts, and irregularly shaped fornices (Figure 3a–e).
Brain MRI of subject IS10-017a1 showing thinning of the splenium of the corpus callosum (arrowhead) and cerebellar vermis hypoplasia (arrow) on T1 sagittal (a). The loculated nature of the posterior fossa cysts can be seen on T1 axial (b and c; arrows). The pillars of the fornix were asymmetric, as seen on T1 axial (d; arrow) and T1 coronal (e; arrow) views. Her sister, subject IS10-017a2, had similar findings of partial agenesis of the corpus callosum (arrowhead) and cerebellar vermis hypoplasia on T1 sagittal (f). The posterior fossa cysts were similarly loculated on T2 axial views (g and h; arrows). The pillars of the fornix appeared normal on T2 axial (i; arrow) and T1 coronal (j; arrow) views.
Subject IS10-017a2 (Figure 1c and d), the younger sister of subject IS10-017a1 (Figure 1e), was 7 years old at the time of study. She presented at birth with ambiguous genitalia, described as hyper-rugated labia majora but with a small clitoris. The anus was described as posteriorly placed. Evaluation at the time including abdominal ultrasound, karyotype, cortisol, 11-deoxycortisol, DHEA-S, free and total testosterone, and 17-hydroxy-progesterone levels were unremarkable. At 4 months of age she developed infantile spasms with upward eye deviation and bilateral arm extension. EEG showed high voltage and multifocal epileptiform discharges consistent with hypsarrhythmia, and electrodecrement coinciding with epileptic spasms (Figure 2c). She also developed intractable epilepsy (Figure 2d) with EEG features of Lennox–Gastaut syndrome. The details of her epilepsy course are found in Supplementary Data.
Developmentally at age 7 years she was able to sit independently, roll, rise off the floor on all four extremities but was non-ambulatory. She did not visually regard or track. She had no verbal language, made vocalizations, and had frequent inappropriate laughter and crying. She was not able to feed herself. Physical exam was notable for weight 40th percentile for age, head circumference at the 98th percentile, mild hypertelorism, and scoliosis. Brain MRI (Figure 3f–j) showed partial agenesis of the corpus callosum (absent splenium), and cerebellar vermis hypoplasia, with posterior fossa cysts. The pillars of the fornix were normal.
The time course of epilepsy in both subjects is summarized in Table 1. IS10-017a2 underwent multiple normal genetic studies, including chromosomal microarray, ARX and CDKL5 sequencing, serum amino acids, urine organic acids, serum and cerebral spinal fluid lactate, pyruvate, amino acids, and neurotransmitters, serum acylcarnitine profile, leukocyte lysosomal enzymes, very-long-chain fatty acids, carbohydrate-deficient glycoprotein, urine sulfites, creatine, purine, and pyrimidine metabolites. A skeletal survey demonstrated 11 ribs bilaterally. An electroretinogram at age 15 months was performed because of poor visual tracking, and was equivocal for cone rod dystrophy. Visual-evoked potentials were normal.
Whole exome sequencing
We performed WES of blood cell-derived genomic DNA from subject IS10-017a1 with the hypothesis that her neurodevelopmental disorder was shared with her affected sibling in an autosomal recessive manner. Mean depth of coverage over the targeted exome was 105 × . Our analysis identified variants in two genes fitting an autosomal recessive model: a homozygous variant (chr17: g.66347790 C>T) in ARSG and compound heterozygous variants (chr7: g.98782656 C>G and chr7: g.98782671 G>C) in KPNA7. Depth of coverage was 74 × for the chr7: g.98782656 C>G variant and 76 × for the chr7: g.98782671 G>C variant in KPNA7. The variants in both genes were absent from control dbSNP databases and >5500 exomes available in the Exome Variant Server, suggesting they were rare and not found in the normal population. A search of more than 700 gene–phenotype associations in DBDB 1.2 did not find other variants overlapping with known genes for neurodevelopmental disorders, particularly when autosomal recessive inheritance was considered.
Sanger sequencing
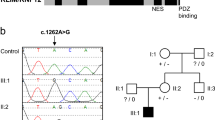
We confirmed the ARSG and KPNA7 variants by Sanger sequencing both of the affected subjects and both parents. The ARSG variant was confirmed homozygous in IS10-017a1, but was found to be heterozygous in IS10-017a2 and in both parents. As this did not support an autosomal recessive mode of inheritance, we excluded ARSG from further consideration. However, the KPNA7 mutations were found in a compound heterozygous state in both affected subjects, as well as heterozygous the mother (c.1015C>G (p.(Pro339Ala))) and father (c.1030G>C (p.(Glu344Gln))) consistent with an autosomal recessive disease model (Figure 4). We then performed Sanger sequencing in the unaffected siblings, and did not find either of the variants present. The coding regions and intron/exon boundaries of KPNA7 were next sequenced in a cohort of 56 sporadic infantile spasms and 16 cerebellar vermis hypoplasia subjects with epilepsy, but did not find any additional mutations.
Compound heterozygous mutations c.1015C>G and c.1030G>C in KPNA7 identified by WES of subject IS10-017a1 were confirmed by Sanger sequencing in both of the affected subjects and parents. The c.1015C>G (p.(Pro339Ala)) mutation (a) and the c.1030G>C (p.(Glu344Gln)) mutation (b) were present in both affected subjects in a compound heterozygous manner. The c.1015C>G (p.(Pro339Ala)) mutation was carried by the mother and the c.1030G>C (p.(Glu344Gln)) mutation was carried by the father. Neither mutation was found in the unaffected siblings.
Localization of the disease-associated amino-acid substitutions within the predicted 3D structure of KPNA7
KPNA7 encodes a member of the importin α nuclear import receptor family that, based on its structural and biochemical properties, likely mediates translocation of NLS-containing proteins from the cytoplasm to the nucleus.20 The amino-acid sequence of KPNA7 is 43% identical to that of KPNA2, and the latter has been studied extensively including at the structural level.17 The tertiary structure of KPNA2 is formed from stacking a series of three-helix structures known as ARM repeats that together form a concave groove for NLS binding.21 The two clusters of basic residues within a bipartite NLS contact two sites in KPNA2, a major site (ARM repeats 2–4) and a minor site (ARM repeats 6–8).22, 23 We threaded KPNA7 onto the crystal structure of KPNA2 (PDB ID: 1PJM)17 and found the KPNA7 Pro339Ala/Glu344Gln substitutions were part of helix 3 of ARM repeat 7. This placed the amino-acid changes proximal to the site that binds the second element of the bipartite NLS of Rb (Figure 5a). Pro339 in KPNA7 is not in a conserved position, although all other KPNA family members have a proline in the same loop, between helix 2 and helix 3 of the seventh ARM repeat. It is possible that the bend in the carbon backbone of the loop conferred by proline is necessary for the proper placement of helix 3, but that its exact position in the loop is not important. Glu344 is conserved in all family members (Figure 5b), so we used KPNA2 as a model to assess how this residue affects NLS binding. The Glu354Gln substitution in KPNA2 reduced binding to the Rb bipartite NLS to approximately 50% of wild-type (WT) KPNA2 (range 49–57%)(Figure 5c). On the basis of these data, the disease-associated amino-acid substitutions in KPNA7 are predicted to reduce NLS-binding activity of cargoes that interact with the minor site that includes ARM repeat 7.
Amino-acid substitutions in the nuclear import receptor KPNA7 map to a helix implicated in NLS binding. (a) Ribbon structure of KPNA2 (green) with the bipartite NLS from Rb (red) generated using PDB 1PJM. The KPNA7 model (purple) was produced by threading the KPNA7 sequence onto IPJM. The amino-acid substitutions identified in the affected subjects are indicated on both models. (b) Sequence alignment from helix 3 of ARM 7 from human KPNA proteins. Positions of amino-acid identity across 5 or more family members are shown in bold, and the KPNA7 substitutions are marked with arrows. (c) Binding assays performed with the bipartite NLS of Rb immobilized on beads and WT and Glu354Gln (E354Q) forms of KPNA2. The full colour version of this figure is available at European Journal of Human Genetics online.
Discussion
We describe two siblings from a non-consanguineous family with autosomal recessive mutations in KPNA7 with a syndrome characterized by infantile spasms, subsequent intractable epilepsy consistent with Lennox–Gastaut syndrome, partial agenesis of the corpus callosum, cerebellar vermis hypoplasia with posterior fossa cysts, and severe neurodevelopmental outcome. To our knowledge, the KPNA7 mutations identified here by WES are the first nuclear transport receptor mutations to be described in a human disease.
We used molecular modeling to demonstrate that the amino-acid substitutions in KPNA7 localized to a region that is highly conserved across KPNA proteins and involved in NLS binding. In the absence of physiological targets of KPNA7, we modeled the effects of the mutations on the closest family member, KPNA2. We found that introducing a single amino-acid substitution (Glu354Gln) in the ARM repeat 7 reduced KPNA2 binding to the bipartite NLS in Rb. We hypothesize that mutations in KPNA7 induce clinical phenotypes because of reduced nuclear import of cargoes carried by this receptor. Given that there are NLS binding preferences displayed by some KPNA family members,4 and KPNA7 is one of the least abundant transport receptors, it is plausible that reduced function of KPNA7 affects nuclear protein import of only a small number of nuclear proteins. If, for example, KPNA7 mutations decrease the nuclear levels of transcriptions factors that are important for GABAergic interneuron development, epilepsy-associated phenotypes would be observed. In this regard, members of the importin-β family, including importin β1 (KPNB1), mediate nuclear transport of homeodomain transcription factors (4), including ARX24. In the mouse KPNA7 may interact with KPNB1.25 Mutations in the nuclear localization sequence of ARX are associated with both infantile spasms and lissencephaly, and result in abnormal subcellular distribution of the protein product.26 As loss-of-function mutations in ARX cause a spectrum of infant-onset epilepsy disorders, this observation further supports the concept that nuclear concentrations of ARX proteins might be compromised by reduced activity of the import machinery.
KPNA7 is currently the least understood KPNA family member, as NLS cargoes specific for this receptor have not been identified. KPNA7 is highly expressed in bovine and porcine ovarian tissues, oocytes, and embryos.27, 28 In mice, truncating mutations resulted in reduced reproductive potential and female lethality.25 These expression patterns are interesting as one of our subjects had ambiguous genital findings both at birth and on subsequent examination, without identifiable endocrine disturbance. The family member KPNA2 is a binding partner of the androgen receptor-interacting protein 3 (Arip3).29 Given the sequence homology between KPNA2 and KPNA7, it is possible that mutations in KPNA7 may also negatively impact androgen receptor signaling during embryonic development.
We sought, but did not find, mutations in KPNA7 in other subjects with infantile spasms and in subjects with epilepsy and cerebellar vermis hypoplasia. All of the additional subjects sequenced, however, were sporadic cases and therefore most likely to be caused by de novo, not autosomal recessive, genetic events. Combined with the absence of our KPNA7 mutations in the dbSNP data sets, this suggests that mutations in KPNA7 are rare causes of neurodevelopmental disorders.
Impairment of forebrain and hindbrain transcription factor function has emerged as an important cause of severe neurodevelopmental disorders of childhood. These disorders feature complex neurologic disability, and most affected children are non-verbal, non-ambulatory, with multiple neurobehavioral impairments. Cerebellar vermis hypoplasia can be an imaging finding in a subset of children with autism,30 and is also seen as part of the Dandy–Walker malformation spectrum in patients with deletions of 6p25.3 that include the hindbrain transcription factor FOXC1.31 The phenotype seen in the two subjects with KPNA7 mutations described here contains elements of both abnormal forebrain and hindbrain development. We speculate that the hindbrain malformation seen in our subjects was mediated by decreased activity of transcription factors important for cerebellar development, due to reduced KPNA7 function. Our data suggest that mutations in KPNA gene family members may be a novel cause of childhood neurodevelopmental disorders.
References
Pemberton LF, Paschal BM : Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 2005; 6: 187–198.
Marfori M, Mynott A, Ellis JJ et al: Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim Biophys Acta 2011; 1813: 1562–1577.
Terry LJ, Shows EB, Wente SR : Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 2007; 318: 1412–1416.
Ye W, Lin W, Tartakoff AM, Tao T : Karyopherins in nuclear transport of homeodomain proteins during development. Biochim Biophys Acta 2011; 1813: 1654–1662.
Major AT, Whiley PAF, Loveland KL : Expression of nucleocytoplasmic transport machinery: clues to regulation of spermatogenic development. Biochim Biophys Acta 2011; 1813: 1668–1688.
Yasuhara N, Shibazaki N, Tanaka S et al: Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol 2007; 9: 72–79.
Strømme P, Mangelsdorf ME, Scheffer IE, Gécz J : Infantile spasms, dystonia, and other X-linked phenotypes caused by mutations in Aristaless related homeobox gene, ARX. Brain Dev 2002; 24: 266–268.
Amiel J, Rio M, De Pontual L et al: Mutations in TCF4, encoding a class I basic helix–loop–helix transcription factor, are responsible for Pitt–Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet 2007; 80: 988–993.
Dastot-Le Moal F, Wilson M, Mowat D, Collot N, Niel F, Goossens M : ZFHX1B mutations in patients with Mowat–Wilson syndrome. Hum Mutat 2007; 28: 313–321.
Le Meur N, Holder-Espinasse M, Jaillard S et al: MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J Med Genet 2010; 47: 22–29.
Ariani F, Hayek G, Rondinella D et al: FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet 2008; 83: 89–93.
Kato M, Dobyns WB : X-linked lissencephaly with abnormal genitalia as a tangential migration disorder causing intractable epilepsy: proposal for a new term, ‘interneuronopathy’. J Child Neurol 2005; 20: 392–397.
Mowat DR, Wilson MJ, Goossens M : Mowat–Wilson syndrome. J Med Genet 2003; 40: 305–310.
Kortüm F, Das S, Flindt M et al: The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J Med Genet 2011; 48: 396–406.
Lux AL, Osborne JP : A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia 2004; 45: 1416–1428.
Paciorkowski AR, Thio LL, Dobyns WB : Genetic and biologic classification of infantile spasms. Pediatr Neurol 2011; 45: 355–367.
Fontes MRM, Teh T, Jans D, Brinkworth RI, Kobe B : Structural basis for the specificity of bipartite nuclear localization sequence binding by importin-alpha. J Biol Chem 2003; 278: 27981–27987.
Guex N, Peitsch MC : SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 1997; 18: 2714–2723.
Arnold K, Bordoli L, Kopp J, Schwede T : The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 2006; 22: 195–201.
Kelley JB, Talley AM, Spencer A, Gioeli D, Paschal BM : Karyopherin alpha7 (KPNA7), a divergent member of the importin alpha family of nuclear import receptors. BMC Cell Biol 2010; 11: 63.
Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA : Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol 2004; 14: 505–514.
Conti E, Uy M, Leighton L, Blobel G, Kuriyan J : Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell 1998; 94: 193–204.
Fontes MR, Teh T, Kobe B : Structural basis of recognition of monopartite and bipartite nuclear localization sequences by mammalian importin-alpha. J Mol Biol 2000; 297: 1183–1194.
Lin W, Ye W, Cai L et al: The roles of multiple importins for nuclear import of murine aristaless-related homeobox protein. J Biol Chem 2009; 284: 20428–20439.
Hu J, Wang F, Yuan Y et al: Novel importin-alpha family member Kpna7 is required for normal fertility and fecundity in the mouse. J Biol Chem 2010; 285: 33113–33122.
Shoubridge C, Tan MH, Fullston T et al: Mutations in the nuclear localization sequence of the Aristaless related homeobox; sequestration of mutant ARX with IPO13 disrupts normal subcellular distribution of the transcription factor and retards cell division. Pathogenetics 2010; 3: 1.
Tejomurtula J, Lee K-B, Tripurani SK, Smith GW, Yao J : Role of importin alpha8, a new member of the importin alpha family of nuclear transport proteins, in early embryonic development in cattle. Biol Reprod 2009; 81: 333–342.
Wang X, Park K-E, Koser S, Liu S, Magnani L, Cabot RA : KPNA7 an oocyte- and embryo-specific karyopherin α subtype, is required for porcine embryo development. Reprod Fertil Dev 2012; 24: 382–391.
Ly-Huynh JD, Lieu KG, Major AT et al: Importin alpha2-interacting proteins with nuclear roles during mammalian spermatogenesis. Biol Reprod 2011; 85: 1191–1202.
Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL : Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 1988; 318: 1349–1354.
Aldinger KA, Lehmann OJ, Hudgins L et al: FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy–Walker malformation. Nat Genet 2009; 41: 1037–1042.
Acknowledgements
We thank the families of our research subjects for sharing details of their children’s condition with us. ARP is supported by K08NS078054, WBD is supported by R01NS058721, and BMP is supported by R01AG040162.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Paciorkowski, A., Weisenberg, J., Kelley, J. et al. Autosomal recessive mutations in nuclear transport factor KPNA7 are associated with infantile spasms and cerebellar malformation. Eur J Hum Genet 22, 587–593 (2014). https://doi.org/10.1038/ejhg.2013.196
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2013.196
Keywords
This article is cited by
-
NPM2 in malignant peritoneal mesothelioma: from basic tumor biology to clinical medicine
World Journal of Surgical Oncology (2022)
-
An epilepsy-associated mutation in the nuclear import receptor KPNA7 reduces nuclear localization signal binding
Scientific Reports (2020)
-
Lessons learned from gene identification studies in Mendelian epilepsy disorders
European Journal of Human Genetics (2016)