Abstract
The spinocerebellar ataxias (SCA) are a genetically and clinically heterogeneous group of diseases, characterized by dominant inheritance, progressive cerebellar ataxia and diverse extracerebellar symptoms. A subgroup of the ataxias is caused by unstable CAG-repeat expansions in their respective genes leading to pathogenic expansions of polyglutamine stretches in the encoded proteins. In general, unstable CAG repeats have an uninterrupted CAG repeat, whereas stable CAG repeats are either short or interrupted by CAA codons, which – like CAG codons – code for glutamine. Here we report on an infantile SCA2 patient who, due to germ-line CAG repeat instability in her father, inherited an extremely expanded CAG repeat in the SCA2 locus. Surprisingly, the expanded allele of the father was an interrupted CAG repeat sequence. Furthermore, analyses of single spermatozoa showed a high frequency of paternal germ-line repeat sequence instability of the expanded SCA2 locus.
Similar content being viewed by others
Introduction
The spinocerebellar ataxias (SCA) are a genetically and clinically heterogeneous group of diseases, characterized by dominant inheritance, progressive cerebellar ataxia and a variety of extracerebellar symptoms. Mutations in 32 different genes have so far been associated with dominantly inherited autosomal SCA.1 SCA 1, 2, 3, 6, 7, 12, 17 and dentatorubral pallidoluysian atrophy (DRLPA) are all caused by unstable CAG-repeat expansions in their respective genes leading to pathogenic expansions of polyglutamine stretches in the encoded proteins, hence the name polyQ diseases.1 The CAG-repeat disorders also share a tendency of anticipation, which is more likely to occur when the mutation is transmitted through the paternal germ line. In general, unstable CAG repeats have uninterrupted CAG motifs, whereas stable CAG repeats are either short or interrupted by CAA codons also coding for glutamine.1, 2, 3 SCA 2, 7, Huntington’s disease and DRLPA can present with extreme expansions in the CAG repeats leading to infantile/juvenile onset of the disease, often displaying different phenotypes.4, 5
Adult-onset SCA2 is characterized by progressive ataxia, extremely slow saccades, dysarthria, supranuclear ophthalmoplegia, cognitive impairment, peripheral neuropathy, as well as action or postural tremor, and the mean age of symptom onset is 30–35 years. The CAG repeat is located on chromosome 12 in the Ataxin-2-gene (ATXN2), and normal alleles have 31 or fewer CAG repeats.1
Only a few infantile SCA2 patients with onset between 2 and 12 months have been reported. They were heterozygous for CAG repeats between 92 and 750 and presented with developmental motor delay, hypotonia, mental retardation and ocular involvement.4, 5, 6
Here we report on an infantile SCA2 patient, who inherited an extremely expanded CAG repeat in ATXN2 from her father due to paternal germ-line CAG repeat instability. Contrary to the general assumption, the expanded allele of the father was an interrupted CAG repeat sequence. Furthermore, analyses of single spermatozoa revealed a high frequency of paternal germ-line repeat sequence instability of the ATXN2 locus.
Materials and methods
Clinical examination
The proband was examined neurologically, and magnetic resonance imaging (MRI) scan and neuropsychological tests were conducted. His daughter was examined clinically and blood samples were obtained and analyzed for metabolic, mitochondrial and lysosomal abnormalities. Furthermore, electroencephalography (EEG) and brain MRI were conducted.
Molecular analysis
DNA was isolated from blood by standard methods. Genotyping of the ATXN2 repeat was performed by fluorescent-labeled primer PCR and the presence of the extremely elongated CAG repeats was tested by triplet repeat primed PCR (TP-PCR). Analyses of the CAG repeat in single cells were conducted using TP-PCR, after isolation of single spermatozoa.7 Several blank controls were included in each run. All fragment-length determinations were performed with capillary electrophoresis on an ABI 3130xl Genetic Analyzer (Applied Biosystems, Nærum, Denmark).
To identify the exact sequence of the CAG/CAA repeats, PCR products containing the ATXN2 CAG/CAA repeats were amplified from genomic DNA, cloned into the PCR2.1 vector (Invitrogen, Nærum, Denmark) and sequenced. In order to exclude the possibility that the sequenced CAG/CAA patterns were caused by an artifact of the PCR amplification, each allele was cloned twice from two different PCR reactions.
Primer information, PCR conditions and so on are available as Supplementary Information.
Results
Clinical report
The proband, a 49-year-old man (II-3, Figure 1), was referred because of gait disturbances and imbalance, difficulties in speaking and slightly decreased tactile sensation of the fingertips. The symptoms had progressed slowly during the last 8 to 10 years.
Pedigree of the SCA2 family. Individuals marked with solid black have a molecular diagnosis of SCA2 accompanied by clinical manifestations. Individuals marked with gray shading have a clinical diagnosis of a neurological disorder that has not been confirmed at the molecular level. The proband is marked by an arrow.
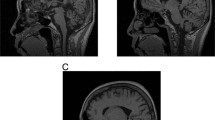
Neurological examination revealed bulging eyes, difficulties in initiating saccades, slow saccades, fasciculations in the face and on the medial side of the thighs, dysarthria and limb ataxia. The gait was wide-based, and he was unable to perform tandem walking (12 on the scale for the assessment and rating of ataxia8). Neuropsychological test revealed problems with concentration and speed, as well as mild executive dysfunction. A brain MRI showed discrete cerebellar atrophy and an enlarged cerebello-medullary cistern (Figure 2). Taken together, this was suggestive of SCA and the patient was subsequently tested positive for SCA2 with CAG/CAA repeat lengths of 22 and 45 in ATXN2.
The family history revealed that the proband’s father (I-1) developed increasing balance and gait problems at the age of 40 and died 30 years later of what at that time was thought to be Alzheimer’s disease. His brother (II-1) was reported to have similar gait disturbances, and his daughter (III-2) had died from multi-organ failure at the age of 19 months.
A detailed review of hospital records revealed that the daughter (III-2), 13 years before the diagnosis of the proband, developed myoclonic jerks, delayed motor milestones and signs of visual impairment at the age of 3 months. She was born at term after a normal pregnancy. At the age of 6 months, she was referred to a local hospital, where she was described as having no or very sparse eye contact, uncoordinated eye movement with parallel eye axes, lack of head control and hypotonia in the upper extremities and the trunk. Furthermore, generalized myoclonic jerks and athetoid movements were noticed. Muscle tone and reflexes were normal. EEG showed bilateral spike foci in the frontal and parietal regions. At the age of 9 months, brain MRI was normal except for a relatively large cerebello-medullary cistern. Metabolic screening showed no sign of inborn errors of metabolism, mitochondrial or lysosomal abnormalities. Antiepileptic therapy and physiotherapy were instituted. At the age of 13 months, she had motor improvement, but still had many dyskinetic movements while awake. Ophthalmologic examination showed delayed visual development, pallor of the optic nerves and hyperpigmentation of dystrophic retinae. A tentative diagnosis of dyskinetic cerebral palsy was assigned. At the age of 17 months, she developed generalized edema and proteinuria, and minimal change glomerulonephritis was diagnosed based on a kidney biopsy. She died of sepsis and multi-organ failure 2 months later despite intensive care treatment. The parents objected to an autopsy, but DNA from peripheral leukocytes was saved for future analyses.
Mutation analyses
The diagnosis of SCA2 in the proband prompted testing of genomic DNA from his daughter. Initial fragment analysis showed apparent homozygosity for a 22-CAG repeat in ATXN2. However, TP-PCR analysis revealed the presence of an expanded allele of at least 124 repeats (Figure 3), and it was concluded that the girl had suffered from infantile SCA2. Cloning and direct sequencing of the proband’s alleles revealed the following CAG/CAA patterns:
Estimation of CAG repeat length in ATXN2 by triplet repeat primed PCR (TP-PCR). The TP-PCR peak profiles (green) show a gradually descending array of peaks. The length of the CAG repeat is estimated from the length of the longest TP-PCR product distinguishable from signal noise (gray-shaded area in the insets). Insets show zooms of the corresponding profiles in order to more precisely estimate the minimal CAG repeat length. Numerical values on the x-axis denote the product size, whereas the y-axis denotes arbitrary units of fluorescence intensity. Size standards are shown in red. (a) Profile from TP-PCR on genomic DNA of the proband. The size of the longest allele of the proband was estimated to be 45 CAG repeats. (b) Profile from TP-PCR on genomic DNA of the proband’s daughter. The size of the longest allele was estimated to be at least 124 CAG repeats. (c, d) Representative results from two isolated single cells of the proband show repeat lengths of about 45 (c) and at least 92 (d).
‘22’: (CAG)8CAA(CAG)4CAA(CAG)8
‘45’: (CAG)36CAA(CAG)8.
Cloning of the girl’s short allele showed a CAG/CAA pattern of (CAG)8CAA(CAG)4CAA(CAG)8. Cloning of the long allele was unsuccessful.
Single-cell sperm analyses
To investigate whether the transmission of the extremely elongated allele was due to a single rare event during meiosis, we analyzed the length of the CAG repeat in ATXN2 in single spermatozoa from the proband by TP-PCR. In 33 single spermatozoa we found CAG repeats ranging from 22 to at least 116 CAGs (Table 1 and Figure 3), and in a healthy control we found alleles with CAG repeat lengths of only 22 and 23 in accordance with results obtained by fragment analyses of genomic DNA from peripheral leukocytes; thus, no signs of meiotic instability were present in the healthy control (Table 1). Finally, single spermatozoa of an unrelated SCA2 patient with repeat lengths of 22 (repeat structure: (CAG)8CAA(CAG)4CAA(CAG)8) and 38 (no CAA interruptions) showed a similar degree of meiotic instability (Table 1), as 4 in 25 cells originating from cells carrying the patient’s 38 allele had long CAG repeats (≥70) in ATXN2.
Discussion
Here we report on a family in which infantile-onset SCA2 was diagnosed several years post-mortem after disease manifestation in her father. Molecular genetic analysis revealed that the infant had a CAG repeat length in ATXN2 of at least 124, expanded from her father’s allele of only 45 CAG repeats. CAG repeat instability has previously been described in a number of polyQ diseases as germ-line instability or intergenerational variations in repeat size.4, 5, 7, 9, 10 However, to our knowledge, analyses of germ-line instability have not been investigated in SCA2. Out of 18 single spermatozoa with the expanded ATXN2 allele, 4 (22%) showed an expansion beyond the 45 CAG repeats detected in somatic cells. Two spermatozoa harbored CAG repeat lengths of at least 92 and 116, respectively. Ninety-two CAG repeats is the shortest repeat known to have caused infantile-onset SCA2.5 A high degree of germ-line instability is also found in SCA1, SCA3, SCA7 and DRPLA. However, in SCA1 and SCA3 the longest gains reported were by 12 and 24 CAG repeats,7, 11, 12 respectively, whereas much longer gains have been reported for SCA7 and DRPLA.9, 10 Exactly why CAG repeats in some genes are gaining more repeats than others remains obscure, but it seems that within a given disease longer repeats tend to be more unstable, as do pure CAG repeats, compared with repeats interrupted by CAA codons.2, 3 The extreme expansion observed in the girl was surprising, given the fact that her father’s pathogenic allele of 45 glutamine codons was interrupted by a CAA codon at position 37. Furthermore, although SCA2 primarily has been associated with pure repeats of more than 35 CAGs13, 14, 15 and interrupted repeats with lengths between 34 and 49 glutamine codons with parkinsonism,16, 17 the proband in our case had a classic SCA2 phenotype. The observation that the CAG expansion in the proband’s interrupted allele has happened prior to the interruption is consistent with previous results showing that the 5′-tract of interrupted repeats is much more prone to expansions than the 3′-CAG tract.3
Upon analyses of sperm from two unrelated SCA2 patients, we found high frequencies of extremely long CAG repeats suggesting that expansion to extreme lengths is a fairly common event. This suggests that such meiotic instability is a general feature of SCA2, although analyses of sperm from more patients are needed to draw firm conclusions in this regard. However, few cases of infantile-onset SCA2 have been reported worldwide, and it is possible that those spermatozoa with very long repeats are dysfunctional or have impaired survival, or that very long repeats are associated with reduced embryonic survival.
In conclusion, we report on an infantile SCA2 patient diagnosed post-mortem after manifestation of the disease in her father. This highlights the importance of considering rare genetic disorders during diagnosis of infants and children even without known neurodegenerative disorders in the family. Furthermore, we show a high degree of germ-line instability in the ATXN2 locus of her father in spite of a CAA interruption in his CAG repeat as well as in an unrelated patient with a pure CAG repeat. Our observations question the general assumption that instability of ATXN2 is primarily limited to pure CAG repeats and that extreme expansion of the CAG repeat is very rare events.
References
Bird TD : Hereditary ataxia overview; in Bird TD, Pagon RA, Dolan CR, et al: (eds): GeneReviews 2nd edn Seattle: University of Washington, 2012.
Chung MY, Ranum LP, Duvick LA, Servadio A, Zoghbi HY, Orr HT : Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet 1993; 5: 254–258.
Choudhry S, Mukerji M, Srivastava AK, Jain S, Brahmachari SK : CAG repeat instability at SCA2 locus: anchoring CAA interruptions and linked single nucleotide polymorphisms. Hum Mol Genet 2001; 10: 2437–2446.
Paciorkowski AR, Shafrir Y, Hrivnak J et al: Massive expansion of SCA2 with autonomic dysfunction, retinitis pigmentosa, and infantile spasms. Neurology 2011; 77: 1055–1060.
Di FR, Santorelli F, Bertini E et al: Infantile childhood onset of spinocerebellar ataxia type 2. Cerebellum 2012; 11: 526–530.
Mao R, Aylsworth AS, Potter N et al: Childhood-onset ataxia: testing for large CAG-repeats in SCA2 and SCA7. Am J Med Genet 2002; 110: 338–345.
Takiyama Y, Sakoe K, Soutome M et al: Single sperm analysis of the CAG repeats in the gene for Machado-Joseph disease (MJD1): evidence for non-Mendelian transmission of the MJD1 gene and for the effect of the intragenic CGG/GGG polymorphism on the intergenerational instability. Hum Mol Genet 1997; 6: 1063–1068.
Schmitz-Hubsch T, du Montcel ST, Baliko L et al: Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 2006; 66: 1717–1720.
Monckton DG, Cayuela ML, Gould FK, Brock GJ, Silva R, Ashizawa T : Very large (CAG)(n) DNA repeat expansions in the sperm of two spinocerebellar ataxia type 7 males. Hum Mol Genet 1999; 8: 2473–2478.
Takiyama Y, Sakoe K, Amaike M et al: Single sperm analysis of the CAG repeats in the gene for dentatorubral-pallidoluysian atrophy (DRPLA): the instability of the CAG repeats in the DRPLA gene is prominent among the CAG repeat diseases. Hum Mol Genet 1999; 8: 453–457.
Koefoed P, Hasholt L, Fenger K et al: Mitotic and meiotic instability of the CAG trinucleotide repeat in spinocerebellar ataxia type 1. Hum Genet 1998; 103: 564–569.
Grewal RP, Cancel G, Leeflang EP et al: French Machado-Joseph disease patients do not exhibit gametic segregation distortion: a sperm typing analysis. Hum Mol Genet 1999; 8: 1779–1784.
Cancel G, Durr A, Didierjean O et al: Molecular and clinical correlations in spinocerebellar ataxia 2: a study of 32 families. Hum Mol Genet 1997; 6: 709–715.
Imbert G, Saudou F, Yvert G et al: Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet 1996; 14: 285–291.
Orr HT, Zoghbi HY : Trinucleotide repeat disorders. Annu Rev Neurosci 2007; 30: 575–621.
Kim JM, Hong S, Kim GP et al: Importance of low-range CAG expansion and CAA interruption in SCA2 parkinsonism. Arch Neurol 2007; 64: 1510–1518.
Charles P, Camuzat A, Benammar N et al: Are interrupted SCA2 CAG repeat expansions responsible for parkinsonism? Neurology 2007; 69: 1970–1975.
Acknowledgements
We would like to thank the family for participating in this study, Helle Andersen from the Laboratory of Reproductive Biology, Copenhagen University Hospital, for her assistance in isolating single spermatozoa and the Novo Nordisk Foundation and the Research Council of Rigshospitalet for funding this work.
Consent and ethics statement: Written informed consent for publication of this report was obtained from all patients alive. The study was approved by the Ethics Committee for the Copenhagen Regional Area, journal no. H-KF-328548.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Vinther-Jensen, T., Ek, J., Duno, M. et al. Germ-line CAG repeat instability causes extreme CAG repeat expansion with infantile-onset spinocerebellar ataxia type 2. Eur J Hum Genet 21, 626–629 (2013). https://doi.org/10.1038/ejhg.2012.231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2012.231
Keywords
This article is cited by
-
From Normal Gait to Loss of Ambulation in 6 Months: a Novel Presentation of SCA17
The Cerebellum (2013)