Abstract
16p11.2 rearrangements are associated with developmental delay, cognitive impairment, autism spectrum disorder, behavioral problems (especially attention-deficit hyperactivity disorder), seizures, obesity, dysmorphic features, and abnormal head size. In addition, congenital anomalies and abnormal brain findings were frequently observed in patients with these rearrangements. We identified and performed a detailed microarray, phenotypic, and radiological characterization of three new patients with 16p11.2 rearrangements: two deletion patients and one patient with the reciprocal duplication. All patients have a heterozygous loss (deletion) or gain (duplication) corresponding to chromosomal coordinates (chr16: 29 528 190–30 107 184) with a minimal size of 579 kb. The deletion patients had language delay and learning disabilities and one met criteria for pervasive developmental disorder not otherwise specified. The duplication patient received a diagnosis of autism and had academic deficits and behavioral problems. The patients with deletion had long cervicothoracic syringomyelia and the duplication patient had long thoracolumbar syringomyelia. The syringomyelia in one patient with deletion was associated with Chiari malformation. Our findings highlight the broad spectrum of clinical and neurological manifestations in patients with 16p11.2 rearrangements. Our observation suggests that genes (or a single gene) within the implicated interval have significant roles in the pathogenesis of syringomyelia. A more comprehensive and systematic research is warranted to study the frequency and spectrum of malformations in the central nervous system in these patients.
Similar content being viewed by others
INTRODUCTION
Recurrent microdeletions and reciprocal duplications of a ∼600 kb genomic region on chromosome 16p11.2 have been associated with a wide spectrum of neurobehavioral abnormalities, including developmental delay/mental retardation, autism spectrum disorder (ASD), behavioral problems (especially attention-deficit hyperactivity disorder (ADHD)), seizures, and schizophrenia.1, 2, 3, 4, 5 More recently, the deletion has also been linked to obesity.6 In our previous cohort of individuals with copy number variants of 16p11.2, we described structural congenital anomalies in ∼30% of deletion and in ∼50% of duplication patients.4 Abnormal brain imaging findings were reported in 9/15 individuals for whom these data were available.4 These included abnormal/prominent CSF spaces (5/15), white matter changes/gliosis (3/15), Chiari I malformation (1/15), and abnormal corpus callosum (1/15). Tethered cord has been reported in one male individual with 16p11.2 microduplication.
Syringomyelia, which is commonly associated with Chiari type I malformation, is a highly heritable condition, as evidenced by familial aggregation, twin studies, and cosegregation with known genetic conditions. Since the early 1900s, multiple families with syringomyelia and Chiari7 I malformation have been reported and autosomal dominant inheritance has been suggested.8 Speer et al9 studied 31 families with two or more individuals affected with Chiari I malformation and syringomyelia. Imaging analysis of asymptomatic first-degree relatives revealed that 21% of those had Chiari I malformation and syrinx,9 supporting a genetic etiology. Genome-wide linkage analyses have attempted to map candidate loci of Chiari I malformation and syringomyelia. The analysis of 71 affected individuals in 23 families identified linkage to loci on 15q21.1–q22.3 and 9q22.31.10
Syringomyelia can be associated with spinal cord tumors, inflammatory arachnoiditis, or spinal injury. Occasionally, no clear etiology for the formation of the syrinx is found (idiopathic syringomyelia). However, the vast majority of cases of syringomyelia are associated with abnormalities at the level of the foramen magnum, particularly Chiari type I malformation, basilar impression, or Dandy–Walker malformation. Chiari type I malformation and syrinx have been reported as being part of several well-established genetic syndromes, including Velocardiofacial syndrome, Shprintzen–Goldberg syndrome, Williams syndrome, achondroplasia, Crouzon syndrome, familial osteosclerosis, and others.7 Some of these syndromes cause bony abnormalities that lead to narrowing of the foramen magnum (eg, achondroplasia and familial osteosclerosis), whereas others affect pathways of axial mesodermal growth and differentiation (eg, Williams syndrome and Shprintzen–Goldberg syndrome). The latter group supports the hypothesis of mesodermal origin for this condition.
In this report, we describe the association of syringomyelia with 16p11.2 rearrangements in three patients. This observation highlights the broad phenotypic variability in patients with 16p11.2 rearrangements and suggests that genes within the implicated interval have significant roles in the pathogenesis of syringomyelia.
Clinical reports
Patient 1
A 16-year-old boy was evaluated in the genetics clinic for a history of syringomyelia and speech and language delays. He was the product of a term, uncomplicated natural delivery with a birth weight of 3.2 kg. The patient was born to non-consanguineous parents of Hispanic Mexican ancestry. Family history was only significant for one paternal first cousin with mild mental retardation of unknown etiology.
The patient was noted to have significant global developmental delays. He began walking at 18 months of age, and two-word phrases were not spoken until the age of 3 years. Currently, at the age of 16 years, he speaks in full sentences, but only a part of it is intelligible. He attends resource classes and functions at a 9- to 12-year-old level. Formal psychological testing has not been obtained.
At the time of evaluation by his geneticist (KA), the patient was noticed to be morbidly obese with a body mass index of 36.6, a height of 170 cm (25–50th centile), weight of 106.1 kg (>99th centile), and head circumference of 59 cm (>98th centile). Mild dysmorphic features of prominent eyes and hypotonic facial expressions and significant fifth finger clinodactyly were also observed. On neurological examination, the patient was noted to have significant dysarthria.
Patient 2
This 14-year-old Caucasian boy was referred to our genetics clinic (CS and MS) for evaluation of weakness and sensory deficits in his lower extremities. He was born to non-consanguineous parents as the product of a term, uncomplicated pregnancy with a birth weight of 3.3 kg. Family history was significant for a paternal first-degree cousin with learning deficits, ADHD, and lower extremity weakness; a 59-year-old paternal grandmother with a history of Chiari I malformation; and a 60-year-old paternal grandfather with a history of spina bifida and seizures. The patient had normal motor and language developmental milestones; however, he experienced delay in toilet training, which was not fully acquired until 8 years of age. He began receiving speech therapy at 5 years of age for dysarthria and hypernasal speech and underwent soft palate repositioning surgery at the age of 7 years. Through the public school system, he was diagnosed with learning disability and central auditory processing disorder in second grade and received occupational therapy from the second to the seventh grade. He reportedly had a 1-year history of weakness in his bilateral legs (motor regression) with difficulty running and walking up stairs. He had intermittent sensory deficits with numbness in his right lower leg approximately 2–3 months before his visit.
On physical examination, the patient's height was 158 cm (50–75th centile), weight 43.5 kg (25–50th centile), and head circumference 55 cm (50–75th centile). The face was mildly dysmorphic with a prominent bulbous nose, mild synophrys, and mild overfolding of bilateral helices (Figure 1a). There was bilateral fifth finger clinodactyly. The neurological examination was significant for mild horizontal nystagmus on extreme lateral gaze, dysarthria, mild decrease of muscular strength in bilateral hip flexors, and brisk lower extremity reflexes with 3/4 on bilateral knees and 4/4 in bilateral Achilles tendons with a 2–3 beat non-sustained clonus bilaterally.
At 14 years of age, the patient underwent a neuropsychological evaluation. Although he had a range of language abilities, there was significant dysarthria and his speech was hypernasal, rapid, and choppy. His domain scores on Module 3 of the Autism Diagnostic Observation Schedule (ADOS) are shown in Table 1. Table 2 presents the results of his psychocognitive testing using the following instruments: Differential Ability Scales – Second Edition (DAS-II) (this instrument is standardized to a mean of 100 with a SD of 15), Vineland Adaptive Behavior Scales, Second Edition (VABS-II), Purdue Pegboard, and Peabody Picture Vocabulary Test – 4th Edition (PPVT-4). The patient's mother completed the Autism Diagnostic Interview-Revised (ADI-R) and, on the basis of her description, he met diagnostic cutoffs for autism in the Reciprocal Social Interaction and Communication domains; however, he did not meet diagnostic cutoffs in the Restricted, Repetitive, and Stereotyped Patterns of Behavior domain (see Table 1). Given the additional information that developmental difficulties were not observed until his third birthday, this patient received a final diagnosis of Pervasive Developmental Disorder – Not Otherwise Specified (PDD-NOS).
Patient 3
This Caucasian male was born at full term by C-section with a birth weight of 3.6 kg. The family history was significant for Charcot–Marie–Tooth disease on the paternal side of the family (with the patient's father and paternal grandmother being affected). The patient was found to carry the PMP22 duplication by fluorescence in situ hybridization analysis. The maternal side of the family included a strong history of psychiatric disorders: mother with anxiety disorder; obsessive-compulsive disorder and trichotillomania in the maternal grandfather; a history of mood swings and abnormal behaviors in the maternal half-sister; and ADHD, mood swings, and aggressive behaviors in the maternal half-niece. The patient was noted to show limited social interests since early infancy. He had a history of developmental delays, including a significant language regression. He used single words meaningfully by 10 months of age but subsequently lost all language skills. These skills returned by approximately 3 years of age, and he used meaningful phrases by 39 months of age. He began walking at the age of 17 months with an unusual, dragging gait, for which he was evaluated by an MRI of his spine (see below). At 3 years 7 months, he was referred for evaluation through the Clinic for Autism Spectrum Disorders at Texas Children's Hospital, where it was determined that he met criteria for autistic disorder, developmental coordination disorder, and a severe receptive/expressive language disorder. His cognitive functioning at that time was evaluated using the Stanford Binet Intelligence Scales – 5th Edition (SB-V)*, and his scores fell within the mild mental retardation range. He met diagnostic cutoffs for autism on both the ADOS-Module 2 and the ADI-R.
He presented to Texas Children's Hospital as a participant in a separate research study at the age of 7 years for evaluation of his behavioral phenotypes, including autism, ADHD, and anxiety disorder. On physical examination, his height was 124 cm (50–75th centile), weight was 25 kg (50–75th centile), and head circumference was 51.9 cm (50th centile). He was not grossly dysmorphic, but had widely spaced teeth, low-set nipples, and a shawl scrotum. His cranial nerves appeared intact. He had normal muscular tone. Deep tendon reflexes were 1/4 in both knees and in Achilles tendons. His gait appeared spastic, with significant toe walking.
Current neuropsychological testing results are shown in Table 2 and indicated that he had made significant progress in terms of his cognitive functioning (as measured by the DAS-II). His significantly weaker performance on the spatial reasoning tasks may have been impacted by his weak fine-motor coordination, as he performed well below normal limits on all trials of the Purdue Pegboard. His mother completed the ADI-R and, on the basis of her description, he met diagnostic cutoffs for autism in Reciprocal Social Interaction; Communication; Restricted, Repetitive and Stereotyped Patterns of Behavior; and age of onset (see Table 1). Evaluation with the ADOS-Module 3 indicated continued deficits in the quality of his reciprocal social communication (see Table 1). On the basis of these results, he continued to meet diagnostic criteria for autistic disorder.
MRI findings
At the age of 14 years, patient 1 experienced generalized weakness, nausea with emesis, and weight loss. A brain MRI revealed Chiari I malformation with cerebellar tonsillar herniation through the foramen magnum to about 1 cm beyond its borders. This was associated with extensive cervicothoracic syringomyelia and mega cisterna magna. He subsequently underwent suboccipital craniectomy with duraplasty and C1 laminectomy.
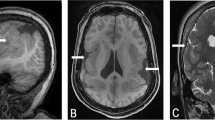
Brain MRI of patient 2 revealed mild foreshortening of the corpus callosum, but no other structural abnormalities. MRI of the spine showed syringohydromyelia from C5 level to the T10 level, measuring 2 mm in greatest diameter opposite T8 (Figures 2a and b). No Chiari malformation, low tethered cord, thickened fatty filum, contrast-enhancing lesions, or other dysraphic features were observed.
MRI findings. (a) and (b) Patient 2 with deletion 16p11.2, (c–f) patient 3 with duplication 16p11.2. (a) Sagittal T2-w imaging of the spine of patient 2 showing dilated central canal extending from C5-T10. No Chiari malformation, low tethered cord, thickened fatty filum, or other dysraphic features were demonstrated. (b) Axial T2-w images demonstrate maximal dilation of the central canal opposite T8. (c) Sagittal T1-w image of patient 3 demonstrating syrinx extending from the cervicothoracic junction down to the conus. No associated Chiari I or other dysraphic features. (d) Sagittal T2-w image confirming extensive syrinx most marked at the thoracolumbar junction, where it returns T2 bright signal. (e) Axial T2-w image at the level of the cervicothoracic junction demonstrating dilatation of the central canal. (f) Axial T1-w image demonstrating maximal dilatation of the extensive syrinx opposite the T12-L1 level. The conus terminates in a normal location and no thickened fatty filum or intrathecal lipoma is observed.
Spine MRI of patient 3 demonstrated a long syrinx from the cervicothoracic junction down to the conus, with maximal dilatation opposite the T12-L1 level. There was no Chiari I malformation or other dysraphic features (Figures 2c–f).
SUBJECTS AND METHODS
Patients and DNA samples
All three cases were referred to the Medical Genetics Laboratories, Baylor College of Medicine (BCM), Houston, USA, for clinical array comparative genomic hybridization (aCGH) analysis. DNA was extracted from whole blood using the Puregene DNA extraction kit (Gentra, Minneapolis, MN, USA) according to the manufacturer's instructions. The study was approved by the Institutional Review Board of BCM, and all participants or parental guardians were recruited after informed consent was obtained. Photographs of the patients were collected after obtaining informed consent for publication of the photographs in the medical literature.
Array CGH
Custom microarrays CMA V6.5 and V8 OLIGO were manufactured by Agilent Technologies (Santa Clara, CA, USA). These arrays contained the best-performing oligos from Agilent’s electronic library and probes for nearly all known microdeletion and microduplication syndrome regions, as well as pericentromeric and subtelomeric regions as previously described.11 The procedures for DNA digestion, labeling, and hybridization for the oligo arrays were performed according to the manufacturer’s instructions, with modifications as in reference 11. Slides were scanned into image files using the Agilent G2565 Microarray Scanner. Scanned images were quantified using Agilent Feature Extraction software (v9.0), then analyzed for copy number change using our in-house analysis package, as described previously.11
FISH analysis
Deletion or duplication was confirmed in all three cases by FISH. Bacterial artificial chromosome RP11-301D18 was used as a probe specific for the respective region (chr16: 29 683 644–29 869 247). Probes were labeled by nick translation using Spectrum Red and Spectrum Green Vysis dUTPs (Vysis, Des Plaines, IL, USA). Hybridization was performed according to the manufacturer's protocol (Abbott Molecular, Abbott Park, IL, USA). Parental peripheral blood samples were obtained and investigated using the same FISH probes.
RESULTS
The clinical aCGH revealed a deletion (patients 1 and 2) or duplication (patient 3) corresponding to chromosomal coordinates (chr16: 29 528 190–30 107 184). In addition, the CMA in patient 3 revealed a paternally inherited duplication in 17p12 (minimal size 1.294 Mb, including the PMP22 gene). All aCGH clinical studies were confirmed by FISH analysis; the deletion was confirmed to be de novo in patient 2, and the 16p11.2 duplication was maternally inherited in patient 3. In patient 1, the mother was negative for the deletion but the father was unavailable.
Clinical findings
Both patients with microdeletions of 16p11.2 presented with weakness and sensory deficits, for which they underwent MRI examination of brain and spine, revealing long cervicothoracic syringomyelia, which was associated with Chiari I malformation in patient 1 and idiopathic in patient 2. Both deletion patients had significant dysarthria and speech delay. Although neither of them met strict diagnostic criteria for autistic disorder, patient 2 was found to meet criteria for PDD-NOS.
Patient 3 with duplication of 16p11.2 has a prominent neurobehavioral phenotype, with a diagnosis of autism, ADHD, and anxiety disorder. He underwent MRI examination of brain and spine for abnormal gait, which revealed extensive thoracolumbar syringomyelia, considered to be idiopathic in the absence of Chiari I malformation, room occupying intraspinal lesions, or a history of trauma.
DISCUSSION
To our knowledge, this is the first study describing the association of 16p11.2 rearrangements with syringomyelia. Our findings highlight the broad spectrum of clinical and neurological manifestations in patients with 16p11.2 rearrangements. The variable expressivity in these subjects may relate to polymorphisms or mutations on the remaining hemizygous allele, different genetic factors elsewhere in the genome, or sex- and age-dependent penetrance for specific traits. The main clinical manifestations in our patients were language delay, mental retardation, and ASD. In addition, all three patients displayed lower extremity spasticity and gait disturbance. The presence of these neurological findings in patients with 16p11.2 rearrangements should prompt clinicians to order spine imaging to rule out syringomyelia.
Congenital malformations have been reported in 30–50% of patients with copy number variants of 16p11.2.4 Structural brain malformations have a high incidence among patients with the 16p11.2 deletion, but congenital anomalies of the spinal cord have not been described previously. Reports of congenital malformations associated with 16p11.2 rearrangements not only have implications for expanding the differential diagnosis but also for anticipatory guidance and establishment of surveillance guidelines for patients with these copy number variants.
Syringomyelia represents a fairly common structural malformation of the spinal cord with an estimated prevalence of 1/1200–1/1500 individuals.7 It is associated with Chiari I malformation in the vast majority of cases. Idiopathic syringomyelia is rarely seen. Our data demonstrate the dosage effect of 16p11.2 and suggest the presence of dose-sensitive genes within the rearranged interval that are important in the pathogenesis of syringomyelia. It is important to note that, in addition to causing autosomal dominant phenotypes, the deletion of genes can occasionally unmask a mutation in the second allele, resulting in an autosomal recessive phenotype, or could cause an imprinting disorder because of deletion of imprinted genes. The former clinical scenario has been described in a patient with severe combined immunodeficiency due to Coronin-1A (MIM 605000) mutation and a 16p11.2 deletion.12
The rearranged 16p11.2 interval contains 27 annotated genes, some of which are potential candidates for the various phenotypes in patients with these copy number changes. The TBX6 (MIM 602427) gene encodes a transcription factor important in developmental processes and can have a role in the congenital spinal anomalies that we observed in our patients.
In conclusion, our data expand the spectrum of phenotypic abnormalities in patients with the 16p11.2 deletion or duplication and suggest candidate loci for spine malformations within the rearranged interval. We also confirm previous observations regarding the association of 16p11.2 with speech/language delay, behavioral abnormalities, and cognitive impairment. The phenotypic variability in patients with 16p11.2 rearrangements may be related to other genetic or genomic variants in the second allele or in unlinked loci, but further molecular analysis is essential to clarify this point. Furthermore, more comprehensive and systematic research is warranted to study the frequency and spectrum of malformations in the central nervous system in patients with copy number variants of 16p11.2 and to determine whether this association is causally related to this chromosomal rearrangement.
References
Weiss LA, Shen Y, Korn JM et al: Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med 2008; 358: 667–675.
Kumar RA, KaraMohamed S, Sudi J et al: Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet 2008; 17: 628–638.
McCarthy SE, Makarov V, Kirov G et al: Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet 2009; 41: 1223–1227.
Shinawi M, Liu P, Kang SH et al: Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioral problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet 2009; 47: 332–341.
Shimojima K, Inoue T, Fujii Y, Ohno K, Yamamoto T : A familial 593-kb microdeletion of 16p11.2 associated with mental retardation and hemivertebrae. Eur J Med Genet 2009; 52: 433–435.
Walters RG, Jacquemont S, Valsesia A et al: A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature 2010; 463: 671–675.
Speer MC, Enterline DS, Mehltretter L et al: Type I malformation with or without syringomyelia: prevalence and genetics. J Genet Couns 2003; 12: 297–311.
Zakeri A, Glasauer FE, Egnatchik JG : Familial syringomyelia: case report and review of the literature. Surg Neurol 1995; 44: 48–53.
Speer MC, Gerge TM, Enterline DS, Franklin A, Wolpert CM, Milhorat TH : A genetic hypothesis for Chiari I malformation with or without syringomyelia. Neurosurg Focus 2000; 8: E12.
Boyles AL, Enterline DS, Hammock PH et al: Phenotypic definition of Chiari type I malformation coupled with high-density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15. Am J Med Genet A 2006; 140: 2776–2785.
Ou Z, Kang SH, Shaw CA et al: Bacterial artificial chromosome-emulation oligonucleotide arrays for targeted clinical array-comparative genomic hybridization analyses. Genet Med 2008; 10: 278–289.
Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, Puck JM : Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a Coronin-1A mutation and a chromosome 16p11.2 deletion. Clin Immunol 2009; 131: 24–30.
Acknowledgements
We thank the patients and parents for their willingness to participate in our research study. We thank Ms Morgan Lasala for assistance in recruiting the patients to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Schaaf, Dr Goin-Kochel, Dr Nowell, Dr Patel, and Dr Bacino are faculty members in the Department of Molecular and Human Genetics at Baylor College of Medicine, Houston, USA, which offers extensive genetic laboratory testing, including the use of arrays for genomic copy number analysis, and the department derives revenue from this activity.
Rights and permissions
About this article
Cite this article
Schaaf, C., Goin-Kochel, R., Nowell, K. et al. Expanding the clinical spectrum of the 16p11.2 chromosomal rearrangements: three patients with syringomyelia. Eur J Hum Genet 19, 152–156 (2011). https://doi.org/10.1038/ejhg.2010.168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2010.168
Keywords
This article is cited by
-
Prenatal microarray analysis in right aortic arch—a retrospective cohort study and review of the literature
Journal of Perinatology (2018)
-
Exome sequencing identifies pathogenic variants of VPS13B in a patient with familial 16p11.2 duplication
BMC Medical Genetics (2016)
-
A highly penetrant form of childhood apraxia of speech due to deletion of 16p11.2
European Journal of Human Genetics (2016)
-
Autism Spectrum Disorder, Developmental and Psychiatric Features in 16p11.2 Duplication
Journal of Autism and Developmental Disorders (2016)
-
Neuroimaging Endophenotypes in Animal Models of Autism Spectrum Disorders: Lost or Found in Translation?
Psychopharmacology (2014)