Abstract
Large-scale copy number variation that is cytogenetically visible in normal individuals has been described as euchromatic variation but needs to be distinguished from pathogenic euchromatic deletion or duplication. Here, we report eight patients (three families and two individuals) with interstitial deletions of 9q13–q21.12. Fluorescence in situ hybridisation with a large panel of BACs showed that all the deleted clones were from extensive tracts of segmentally duplicated euchromatin, copies of which map to both the long and short arms of chromosome 9. The variety of reasons for which these patients were ascertained, and the phenotypically normal parents, indicates that this is a novel euchromatic variant with no phenotypic effect. Further, four patients with classical euchromatic variants of 9q12/qh or 9p12 were also shown to have duplications or triplications of this segmentally duplicated material common to both 9p and 9q. The cytogenetic boundaries between the segmentally duplicated regions and flanking unique sequences were mapped to 9p13.1 in the short arm (BAC RP11-402N8 at 38.7 Mb) and to 9q21.12 in the long arm (BAC RP11-88I18 at 70.3 Mb). The BACs identified in this study should in future make it possible to differentiate between clinically significant deletions or duplications and euchromatic variants with no established phenotypic consequences.
Similar content being viewed by others
Introduction
Segmental duplications (SDs) make up as much as 5% of the human genome and are particularly enriched at pericentromeric and pro-telomeric sites.1 SDs that are >90% identical at the sequence level vary in size from several to hundreds of kilobases in length and can be both subject to, and mediate, large-scale copy number variation.2, 3, 4 Indeed, the common pericentromeric inversion of chromosome 9 is believed to be mediated by homology between the large SDs that flank the heterochromatin of chromosome 9.5, 6, 7 Large-scale copy number variation that is observable at the cytogenetic level has been described as euchromatic variation and euchromatic variants of 8p23.1, 9p12, 9q12/qh, 9q13, 15p11.2 and 16p11.2, which extend to several megabases (Mb), have been reported in multiple families without phenotypic consequences (see the Chromosome Anomaly Collection at http://www.ngrl.org.uk/Wessex/collection/).8
The euchromatic variants of bands 9p12, 9q12/9qh and 9q13 usually involve additional G-band dark, C-band negative, whole chromosome 9 paint-positive euchromatin. The 9p12 euchromatic variant was first described by Buckton et al9 and involves duplication of ∼5 Mb10 or amplification of ∼1 Mb11 of segmentally duplicated material that maps to 9p and 9q. The 9q12/9qh euchromatic variant was first reported by Madan12 and later by others.5, 6, 13, 14, 15, 16 It has been proposed that these 9q12/qh euchromatic variants are derived from 9p125 or 9q13–q21.1.6 The euchromatic variant of 9q13 was originally reported by Jalal et al17 and others18, 19 but has not been further characterised.
This paper describes the characterisation of a new euchromatic deletion variant of 9q13–q21.12 and clarifies the underlying basis of the euchromatic variants of 9q12/qh. It was necessary to map the boundaries between unique and segmentally duplicated euchromatin in 9p and 9q in order to show that all these euchromatic variants involve segmentally duplicated material.
Materials, methods and conventional cytogenetic results
Conventional cytogenetics
Conventional G (GTL) (550 bands) and C (CBG) banding was performed using standard techniques on peripheral blood lymphocytes.
Families 1–3 and patients 1 and 2 with apparent proximal 9q deletions:
Family 1
This 9-year-old boy was referred for chromosome analysis because of learning difficulties and small genitalia. An interstitial deletion of 9q13–q21.12 was found in the proband and his father who was also considered to suffer from cognitive impairment (Figure 1, column 1).
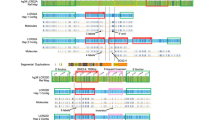
(Column 1) Results from patients with 9q13–q21.12 euchromatic deletion variants. Row 1: Note absence of the most proximal G-dark band q21.11 in the long arm. Row 2: Note also the absence of long arm signals in normal and variant chromosomes with BAC RP13-198D9 that defines the short boundary between segmentally duplicated and unique sequence euchromatin. Row 3: Note the absence of long arm signal on the variant chromosome and the presence of reduced signal strength on the normal 9 of the pSD detected by BAC RP11-246P17. Row 4: Note normal result with segmentally duplicated clone RP11-561O23. Row 5: Note very weak short arm signal that defines the long arm boundary between segmentally duplicated and unique sequence euchromatin with clone RP11-88I18. (Column 2) Results from a patient with the 9q13–q21.12 euchromatic duplication variant and a single extra euchromatic band. Row 1: Note presence of additional G-dark band in q12 in the long arm heterochromatin. Row 2: Single short arm signals in the normal and variant chromosomes with the boundary BAC RP13-198D9. Row 3: Weaker long arm signals on both normal and variant chromosomes of the pSD detected by clone RP11-246P17. Row 4: Extra weaker signal just above the strong long arm signal on the variant chromosome with the segmentally duplicated clone RP11-561O23. Row 5: Weak p arm signal and probable extra band just above strong long arm signal on the variant chromosome with boundary clone RP11-88I18. (Column 3) Results from a patient with 9q13–q21.12 euchromatic triplication variant and two extra euchromatic bands. Row 1: Note presence of two additional G-dark bands in q12 in the long arm heterochromatin. Row 2: Single short arm signals in normal and variant chromosomes with the boundary BAC RP13-198D9. Row 3: Weaker long arm signals on both normal and EV chromosomes of the pSD detected by clone RP11-246P17. Row 4: Two extra relatively weak signals within the chromosome 9 heterochromatic block with segmentally duplicated clone RP11-561O23. Row 5: Weak p arm signal stronger q arm signal and two extra but relatively weak signals within the chromosome 9 heterochromatic block with boundary clone RP11-88I18. (Column 4) Results from the patient with the 9p11.2–p13.1 euchromatic duplication variant and a single extra euchromatic band in the short arm. Row 1: Note presence of additional G-dark band in p12 in the short arm. Row 2: Single short arm signals in normal and variant chromosome with the boundary BAC RP13-198D9. Row 3: Double short arm signals on the variant chromosome, single short arm signal on the normal chromosome and weaker long arm signals on both chromosomes of the pSD detected by clone RP11-246P17. Row 4: Note the double short arm signals on the variant chromosome, single short arm signal on the normal chromosome with SD clone RP11-561O23. Row 5: Note stronger p arm signal on the variant chromosome with boundary clone RP11-88I18.
Families 2 and 3
The male proband in family 2 was referred with global developmental delay at the age of 8. A deletion of 9q13–q21.12 was found in the proband and his phenotypically normal mother. The male proband in Family 3 was referred with learning and behavioural problems and a possible diagnosis of Asperger syndrome at the age of 13. A deletion of 9q13–q21.12 was found in the proband and his phenotypically normal mother.
Patients 1 and 2
Both these male individuals were referred for recurrent miscarriages at the age of 33 and 38, respectively, and were found to have a deletion of 9q13–q21.12. Parental samples were requested but not received.
Patients 3–5 with additional euchromatic band(s) within 9q12/qh:
Patient 3 was ascertained when her mother was referred for a previous trisomy 18 and an increased nuchal translucency of 4.3 mm at 12 weeks gestation conferring a 1 in 7 risk of trisomy 21. Extra material within 9q12/qh was found in chorionic villus cultures but the mother had normal chromosomes 9 and the father was unavailable. The pregnancy resulted in a newborn dysmorphic female and the euchromatic variant was confirmed in an otherwise karyotypically normal perinatal blood sample (Figure 1, column 2).
Patient 4 was a male aged 16 years referred for learning difficulties and obesity. Testing for fragile X and Prader Willi syndromes gave normal results. Extra material was found within 9q12.
Patient 5 was a male ascertained with oligoasthenoteratospermia at the age of 27. Two additional bands were found within the 9q12/qh heterochromatin (Figure 1, column 3). Parental samples were not obtained.
Patient 6 with additional euchromatic band within 9p12:
Patient 6 was a male aged 15 years referred for chromosome analysis with spastic paraparesis and poor balance. An additional G-dark band was observed within 9p12 (Figure 1, column 4). Parental samples were not obtained.
Molecular cytogenetics
Fluorescence in situ hybridisation (FISH) was carried out with build 36.1. Sanger Institute 1 Mb and 37 k cloneset BACs (www.ensembl.org/Homo_sapiens/cytoview) spanning the 9p21 to 9q22 region (Table 1). Bacterial stabs were cultured by standard techniques and minipreps of DNA were made using the Qiagen miniprep kit (www.Qiagen.com). DNA clones were labelled by nick translation with Spectrum Orange or Spectrum Green d-UTP and hybridisation was performed according to the manufacturer's protocols (www.Vysis.com). Dual colour FISH was also carried out with cosmid cCMP9.27 specific for satellite III DNA and BAC RP11-15J10 (Figure 2). Slides were examined with an Olympus BX55 fluorescence microscope and images captured using SmartCapture software (www.digitalscientific.co.uk).
Result from patient with the 9q12 euchromatic variant with a single extra euchromatic band. Note the alternating pattern of green Spectrum Green-labelled satellite III using cosmid cCMP9.27 and red Spectral Orange-labelled signals from the highly segmentally duplicated clone RP11-15J10 illustrated by the additional paralogous sub-telomeric RP11-15J10 signals.
Mapping and gene content
SDs and copy number variations were identified from the Human Genome Segmental Duplication Database (http://projects.tcag.ca/humandup/). The gene content of the segmentally duplicated region was obtained from the Ensembl database (v36) using the MartView data export tool (http://www.ensembl.org/Multi/martview).
Molecular cytogenetic results
FISH on the proband and father from Family 1 gave normal proximal 9q signals with BACs from the Sanger 1 Mb cloneset that mapped to the region of interest between bands q13 and q21.13 according to the Ensembl (http://www.ensembl.org) and NCBI (http://www.ncbi.nih.gov/mapview) web browsers (BACs** in Table 1). However, immediately centromeric to these 1 Mb clones, 37 k tiling path clones including BAC RP11-88I18 gave an additional weak but positive short arm signal, and BAC RP11-561O23 gave strong signals in both the long arm and short arms (Figure 1, column 1). Further BACs that mapped specifically in genome browsers to 9q13, q12, p11.2, p12 or 9p13.1 gave signals in both 9p and 9q in the normal homologue but no signal on the long arm of the euchromatic variant chromosomes (see eg BAC RP11-246P17 in Table 1 and Figure 1, column 1, row 2).
A panel of fully sequenced tiling path BACs was then used to map the extent of this segmentally duplicated pericentromeric region in normal individuals and the results in these and individuals with euchromatic variants are shown in Table 1 (with the extent of the segmentally duplicated region in light grey). All 9p clones telomeric (distal) to RP11-402N8 had signals at a single locus within 9p13 and all 9q clones telomeric (distal) to RP11-88I18 had a single signal within 9q. All of the clones centromeric to these showed signals in both 9p and 9q. This region extends over ∼31.6 Mbs from BAC 402N8 to BAC 88I18 and contains 44 SDs, 17 copy number variants and sequences derived from 93 known and 49 novel genes.
The boundaries between this segmentally duplicated region and flanking unique sequences were within contigs NT_00843.17 in the short arm and NT_023935.17 in the long arm. Cytogenetically, these boundaries lie within the 4 kb overlap between BACs RP13-198D9 and RP11-402N8 in 9p13.1 and within the 118 kb gap between BACs RP11-88I18 and RP11-274B18 in 9q21.12. Centromeric to contig NT_008413.17 in 9p, 17 contigs extending over ∼8 Mb between band p13.1 and the centromere are shown in build 36.1 of the human genome. These contigs each contain between one and 10 BACs. All of the probes tested from these contigs were segmentally duplicated or highly segmentally duplicated (Table 1 and data not shown). Similarly, there are 16 contigs spanning ∼4 Mb between the 9q heterochromatin and NT_023935.17 in the long arm. All of the clones tested from these contigs were either segmentally duplicated or highly segmentally duplicated (Table 1 and data not shown). We considered these SD-containing clones gave three signal patterns:
-
1
partial segmental duplication (pSD in Table 1) with a strong signal in either 9p or 9q and a much weaker signal in the opposite arm.
-
2
SD (in Table 1) with strong signals in both 9p and 9q. Some loci also had additional, clearly separated signals in 9p in addition to the proximal 9q signals (SD+ in Table 1).
-
3
high-level segmental duplication (hSD in Table 1) with signals in both 9p and 9q as well as sub-telomeric 9p and other chromosomes including the ancestral 2q13–q14.1 fusion site at which chromosome 2 was formed in man.20
All eight individuals carrying the euchromatic 9q13–q21.12 deletion variant gave the same results; none of the unique sequence clones were deleted, whereas the long arm signals from the majority of segmentally duplicated clones were consistently absent (‘No’ in Table 1, column 7) except for the 9q21.11/q21.12 clones 561O23 and 88I18 near the distal boundary of the segmentally duplicated region. This variant may be described as var(9)del(9)(q13q21.12).
In the three patients with euchromatic duplication or triplications variants and one or two additional bands within 9q12/qh, most of the segmentally duplicated BACs showed single, double or multiple additional signals (‘Yes’ in Table 1, column 8) (Figure 2). In each duplication and triplication variant, these included the overlapping distal BACs 561O23 and 88I18 but not the four BACs adjacent to the short arm boundary of the segmentally duplicated region (Table 1). These variants may be written as var(9)dup(9)(q13q21.12) or var(9)trp(9)(q13q21.12).
In the single patient with the euchromatic duplication variant and an additional band within 9p12, segmentally duplicated BACs showed two or more additional signals in proximal 9p (‘Yes’ in Table 1, column 9) (Figure 1, columns 1–4). The duplicated tract included BACs from the proximal boundary of the segmentally duplicated region but not the five BACs adjacent to the long arm boundary. This variant may be expressed as var(9)dup(9)(p11.2p13.1).
Discussion
Our results indicate that novel and established euchromatic variants of chromosome 9 all involve deletion, duplication or triplication of large tracts of pericentromeric euchromatin that are themselves composed of segmentally duplicated material. In particular, we have identified and characterised a previously undescribed euchromatic deletion variant of 9q13–q21.12 in three families and two unrelated patients who were all deleted for the same BACs (Table 1). Initially, the phenotype in the father and son from family 1 suggested a causal connection between this deletion variant and the cognitive impairment. However, the segmentally duplicated nature of the deleted region and the presence of the same deletion in the phenotypically normal parents in families 2 and 3, as well as the phenotypically normal individuals 1 and 2 referred with miscarriages, indicate that this is most likely to be a novel euchromatic variant with no phenotypic effect. This conclusion is supported by previous observations, published in abstract,21, 22 of deletion and duplication variants in seven families ascertained for a variety of reasons with a normal parent in each case.
The nature of the extra euchromatic bands in the classical 9q12/9qh euchromatic variants has been previously discussed in the literature with ‘euchromatinisation’ of heterochromatin,12 duplication of an adjacent region23 and amplification of a normally smaller region suggested.16 We have shown here that the 9q12/9qh euchromatic variants are not derived from 9p125 or 9q13–q21.1 alone,6 but result from simple duplication or triplication of large tracts of segmentally duplicated euchromatin that map to both these regions. This is in contrast to the situation on chromosome 16, where duplications of unique sequence proximal 16q are found within the major heterochromatic block and are associated with developmental delay and behavioural problems.24 Finally, we have confirmed the nature and extent of the duplications that underlie the 9p12 euchromatic variants of chromosome 9 which extend over the same area identified by Di Giacomo et al.10 It is interesting that the 9p12 amplification variants reported by Lecce et al11 involve clones at both ends of the 9p12 duplication variant region (Table 1).
The pericentromeric region of chromosome 9 is thought to follow the ‘domain’ model established for chromosome 10 in which the centromere is surrounded by large alphoid repeat arrays which are themselves flanked by interchromosomally duplicated sequences which are, in turn, flanked by intrachromosomal duplications25. Our results are both consistent with and at odds with aspects of this model and the current mapping of chromosome 9 as follows:
-
1
Multiple pericentromeric BAC clones give signals in both the proximal long and short arms of chromosome 9 and cannot, therefore, be assigned to either a single short or long arm location. However, only two of the segmentally duplicated BACs (381O7 and 15J10) have been end sequenced to more than one location in either the same (381O7) or a different chromosome arm (15J10) (Table 1). Thus, the cytogenetic map locations of fully sequenced and fingerprinted clones from this region are clearly not accurately reflected in current genome browsers.
-
2
The boundaries of the segmentally duplicated pericentromeric region are consistent with those of Humphray et al7 at ∼38.7 Mb in the short arm and close to the FOXD4L genes at ∼70.3 Mb in the long arm. However, our results with the euchromatic deletion variant indicate that the segmentally duplicated region extends at least as far as band 9q21.12 in the long arm which is at odds with current genome browsers.
-
3
The whole segmentally duplicated region is itself highly enriched for both large-scale SDs and copy number variations (Table 1 column 12).
-
4
There are no OMIM disease genes within the segmentally duplicated region and only one with an OMIM number (PGM5, OMIM 600981). At present, current mapping indicates that, for example, four contactin-associated protein-like 3 precursor (CNTNAP3) genes map to the pericentromeric short arm and one to the long arm and four COBW domain-containing genes map to the long arm. However, our results imply that additional copies of the genes, pseudogenes and gene fragments from this region will map to both the long and short arms of chromosome 9.
-
5
Pericentromeric BACs did not hybridise within the major heterochromatic block using dual colour FISH with the satellite III probe. Thus, it is likely that the q12 heterochromatic block is a relatively pure array of satellite repeats which is at odds with current genome browsers that assign ∼4 Mb of segmentally duplicated DNA to band q12 (qh).
-
6
We did not find that the high-level interchromosomal segmentally duplicated BACs (hSD in Table 1) were all centromeric to the intrachromosomal segmentally duplicated BACs (SD+ or SD in Table 1) as expected from the 10 centromere model.
Many of these observations reflect the fact that the pericentromeric SDs of chromosome 9 are among the most extensive and complex in the human genome and, as a result, this region has been refractory to sequencing and still contains a large number of sequence gaps.7, 26
It has been proposed in the past that 9q12/9qh euchromatic variants arise from unequal crossing over (non-allelic homologous recombination) at meiosis27 and it is possible that this might be mediated by small segments of satellite DNA which can form the buffer between blocks of SD as observed on chromosome 10.25, 28, 29 The novel deletion euchromatic variants of 9q13–q21.12 might then be the reciprocal product of the duplication euchromatic variants reported in abstract.21, 22 However, such a mechanism is unlikely to explain the more continuous variation found in the 9p12 euchromatic variants described by Lecce et al11 where carriers have between 7 and 12 copies of an ∼1 Mb segment and controls between 1 and 3.
It has also been proposed that these segmentally duplicated regions act as gene nurseries within which new variant genes can evolve from unique ancestors and that copy number variations may have an effect on quantitative traits.25, 30 However, our results and those from previous families, suggest that much of the copy number variation of these regions is phenotypically neutral despite the altered copy number of genes included in the pericentromeric euchromatic variants of chromosome 9.
In conclusion, our results show that deletion, duplication and triplication of segmentally duplicated euchromatin underlies all the pericentromeric euchromatic variants of chromosome 9 including novel deletion variants of 9q13–q21.12 and the established euchromatic variants of chromosome 9q12/qh and 9p12. FISH with the BACs identified in this study should make it possible to differentiate euchromatic variation from pathogenic rearrangements and to obviate the need for parental chromosome analysis in future.
Accession codes
References
Eichler EE : Recent duplication, domain accretion and the dynamic mutation of the human genome. Trends Genet 2001; 17: 661–669.
Sebat J, Lakshmi B, Troge J et al: Large-scale copy number polymorphism in the human genome. Science 2004; 305: 525–528.
Iafrate AJ, Feuk L, Rivera MN et al: Detection of large-scale variation in the human genome. Nat Genet 2004; 36: 949–951.
Sharp AJ, Locke DP, McGrath SD et al: Segmental duplications and copy-number variation in the human genome. Am J Hum Genet 2005; 77: 78–88.
Park JP, Wojiski SA, Spellman RA, Rhodes CH, Mohandas TK : Human chromosome 9 pericentric homologies: implications for chromosome 9 heteromorphisms. Cytogenet Cell Genet 1998; 82: 192–194.
Starke H, Seidel J, Henn W et al: Homologous sequences at human chromosome 9 bands p12 and q13–21.1 are involved in different patterns of pericentric rearrangements. Eur J Hum Genet 2002; 12: 790–800.
Humphray SJ, Oliver K, Hunt AR et al: DNA sequence and analysis of human chromosome 9. Nature 2004; 429: 369–374.
Barber JC : Directly transmitted unbalanced chromosome abnormalities and euchromatic variants. J Med Genet 2005; 42: 609–629.
Buckton KE, O'Riordan ML, Ratcliffe S, Slight J, Mitchell M, McBeath S : A G-band study of chromosomes in liveborn infants. Ann Hum Genet 1980; 43: 227–239.
Di Giacomo MC, Cesarano C, Bukvic N, Manisali E, Guanti G, Susca F : Duplication of 9 p11.2–p13.1: a benign cytogenetic variant. Prenat Diagn 2004; 24: 619–622.
Lecce R, Murdolo M, Gelli G et al: The euchromatic 9p+ polymorphism is a locus-specific amplification caused by repeated copies of a small DNA segment mapping within 9p12. Hum Genet 2006; 118: 760–766.
Madan K : An extra band in human 9qh+ chromosomes. Hum Genet 1978; 43: 259–264.
Berg JM, Gardner HA, Gardner RJM et al: Dic(21;21) in a Down syndrome child with an unusual chromosome 9 variant band. J Med Genet 1980; 17: 144–148.
Docherty Z, Hulten MA : Extra euchromatic band in the qh region of chromosome 9. J Med Genet 1985; 22: 156–157.
Roland B, Cox DM, Hoar DI, Fowlow SB, Robertson AS : A familial interstitial deletion of the long arm of chromosome 21. Clin Genet 1990; 37: 423–428.
Docherty Z, Hulten MA : Rare variant of chromosome 9. Am J Med Genet 1993; 45: 105–106.
Jalal SM, Kukolich MK, Garcia M, Day DW : Euchromatic 9q+ heteromorphism in a family. Am J Med Genet 1990; 37: 155–156.
Knight LA, Soon MG, Tan M : Extra positive band on the long arm of chromosome 9. J Med Genet 1995; 32: 994–995.
Reddy KS : Variants of chromosome 9 with additional euchromatic bands: two case reports. Am J Med Genet 1996; 64: 536–538.
Fan Y, Linardopoulou E, Friedman C, Williams E, Trask BJ : Genomic structure and evolution of the ancestral chromosome fusion site in 2q13–2q14.1 and paralogous regions on other human chromosomes. Genome Res 2002; 12: 1651–1662.
Winters J, Satinover DL, Van Dyke DL, Schwartz S : Molecular and cytogenetic analysis of subtle deletions and duplications in the proximal long arm of chromosome 9: implications for the formation of the common pericentric inversion. Am J Hum Genet 1998; 63 (Suppl): A41.
Mevatee U, Puanita C, Bumroongkit K et al: An additional band on a chromosome 9 with normal phenotype. Chromosome Res 2005; 13 (Suppl 1): 1.33.
Luke S, Verma RS, Conte RA, Mathews T : Molecular characterization of the secondary constriction region (qh) of human chromosome 9 with pericentric inversion. J Cell Sci 1992; 103: 919–923.
Barber JCK, Zhang S, Friend N et al: Duplications of proximal 16q flanked by heterochromatin are not euchromatic variants and show no evidence of heterochromatic position effect. Cytogenetic and Genome Res 2006; 114: 351–358.
Guy J, Hearn T, Crosier M et al: Genomic sequence and transcriptional profile of the boundary between pericentromeric satellites and genes on human chromosome arm 10p. Genome Res 2003; 13: 159–172.
Eichler EE, Clark RA, She X : An assessment of the sequence gaps: unfinished business in a finished human genome. Nat Rev Genet 2004; 5: 345–354.
Macera MJ, Verma RS, Conte RA, Bialer MG, Klein VR : Mechanisms of the origin of a G-positive band within the secondary constriction region of human chromosome 9. Cytogenet Cell Genet 1995; 69: 235–239.
Horvath JE, Bailey JA, Locke DP, Eichler EE : Lessons from the human genome: transitions between euchromatin and heterochromatin. Hum Mol Genet 2001; 10: 2215–2223.
She X, Horvath JE, Jiang Z et al: The structure and evolution of centromeric transition regions within the human genome. Nature 2004; 430: 857–864.
Trask BJ, Massa H, Brand-Arpon V et al: Large multi-chromosomal duplications encompass many members of the olfactory receptor gene family in the human genome. Hum Mol Genet 1998; 7: 2007–2020.
Acknowledgements
We thank all the patients and referring clinicians involved. We should also like to thank Barbara Gorick of the clone Resource Centre at the Sanger Institute for kindly providing the FISH clones used in the study. We also thank Georgina Parkin for her expert technical assistance and the staff of the Cytogenetics laboratories at Cambridge and Guy's Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willatt, L., Barber, J., Clarkson, A. et al. Novel deletion variants of 9q13–q21.12 and classical euchromatic variants of 9q12/qh involve deletion, duplication and triplication of large tracts of segmentally duplicated pericentromeric euchromatin. Eur J Hum Genet 15, 45–52 (2007). https://doi.org/10.1038/sj.ejhg.5201720
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201720
Keywords
This article is cited by
-
Chromosomal microarray analysis as the first-tier test for the identification of pathogenic copy number variants in chromosome 9 pericentric regions and its challenge
Molecular Cytogenetics (2016)
-
Duplication 9p and their implication to phenotype
BMC Medical Genetics (2014)
-
Heteromorphic variants of chromosome 9
Molecular Cytogenetics (2013)