Abstract
CAG repeat expansions with loss of CAT interruptions in the coding region of the ataxin-1 gene are associated with spinocerebellar ataxia type 1 (SCA1). For molecular genetic diagnosis it is necessary to define the limits of normal and pathological size ranges. In most studies, normal alleles as measured by PCR range from 6–39 units with interruptions of 1–3 CAT trinucleotides that are thought to be involved in the stability of the trinucleotide stretch during DNA replication. Expanded alleles have been reported to carry 39–81 CAG trinucleotides without stabilising CAT interruptions. To evaluate the limits between normal and disease size ranges we analysed the repeat length and composition of the SCA1 gene in 15 individuals with alleles ranging from 36 and 41 triplets for genotype-phenotype correlation studies. We found the 39 trinucleotide-allele to be either interrupted by CAT repeats or formed by a pure CAG stretch. The clinical features of individuals carrying 39 uninterrupted CAG repeats did not differ from the SCA1 phenotype in general with dysphagia, pale discs, pyramidal signs and cerebellar tremor being more frequent as compared to other SCA genotypes. In contrast, the interrupted 39 trinucleotide-allele is not correlated with the SCA1 phenotype.
Similar content being viewed by others
Introduction
The spinocerebellar ataxias (SCAs) are autosomal dominant progressive neurodegenerative disorders displaying clinical and genetic heterogeneity. The loci and mutations associated with at least eight subtypes of SCAs have been cloned: SCA11 (MIM 164400), SCA22,3,4 (MIM 183090), SCA35 (MIM 109150), SCA66 (MIM 183086), SCA77 (MIM 164500), SCA88 (MIM 603680), SCA109 (MIM 603516), SCA1210 (MIM 604326). Furthermore, mutations in the DRPLA gene11,12 (MIM 125370) and the TBP/SCA17 gene13,14,15 (MIM 600075) produce SCA-like phenotypes. In these disorders, expansions of unstable nucleotide repeats in the respective genes are implicated in the pathogenesis of the disease. Unstable expansions of trinucleotide repeats have been shown to cause at least 14 neurological diseases (for review).16
Spinocerebellar ataxia type 1 (SCA1) primarily affects the brainstem, spinocerebellar tracts and cerebellar Purkinje cells. Patients with SCA1 develop progressive ataxia of limb and gait, as well as bulbar and pyramidal symptoms. In most cases, there is evidence for fronto-executive dysfunction while general intellectual impairment is restricted to advanced stages of the disease.17,18
The neurodegenerative process in SCA1 is thought to be contingent on transcription and translation of expanded CAG trinucleotide repeats within the coding region of the gene. This results in the synthesis of a pathologically altered gene product harbouring an expanded polyglutamine stretch.1 Normal SCA1 alleles range from 6–39 while clinical symptoms have been reported in individuals carrying 39–81 CAG repeats.1,19,20,21 The age of onset is inversely correlated to the repeat length with large alleles being predominantly found in juvenile cases. Sequence analysis of normal alleles revealed an interrupted repeat configuration with one to three CAT in 98% of the normal chromosomes.22 In contrast, expanded SCA1 alleles are characterised by continuous CAG stretches. Only in rare cases, expanded alleles are interrupted as shown for a patient with 58 repeats and late onset of the disease.23 CAT interruptions are therefore thought to be of critical importance in maintaining the stability of normal SCA1 alleles during DNA replication.
Molecular genetic analysis is performed for clinical diagnosis, predictive or prenatal testing in families with SCA1. Among CAG trinucleotide diseases, Huntington's disease (HD) is characterised by the greatest number of individuals with intermediate alleles and reduced penetrance.24 In SCA1, where normal and disease allele ranges are continuous, reduced penetrance is poorly documented but would be expected in the lower range of expanded alleles. Intermediate alleles and an age-dependent gene penetrance in SCA1 may therefore cause difficulties in interpreting molecular genetic results.25 The aim of the present study was to perform genotype-phenotype correlations in intermediate alleles from 36–41 CAG repeats in the SCA1 gene with respect to the presence of interrupting CAT trinucleotides.
Subjects and methods
Molecular genetic testing
Searching for pathogenic causes in patients with symptoms of ataxia or severe cognitive impairment (differential diagnostic requests) molecular genetic analyses for repeat expansions in the SCA1, SCA2, SCA3, SCA6, SCA7 and SCA12 gene were performed. After having obtained informed consent for genetical analyses, total genomic DNA was extracted from peripheral blood leukocytes by standard protocols.26 After exclusion of known repeat expansions in the genes for SCA2, 3, 6, 7 and 12 the SCA1 CAG repeat was analysed. In 45 unrelated patients, repeat sizes from 42–65 triplets were found. For 15 individuals with (CAG)36 to (CAG)41 detailed molecular genetic analyses of the SCA1 gene were performed.
PCR, restriction and sequence analysis of the CAG repeats in the SCA1 gene
For analysis of repeat length and composition, PCR was performed in a volume of 25 μl containing 50 ng genomic DNA, 10 pmol of each primer, 5 pmol dNTP and 1 units Taq polymerase (Q•BIOgene) with an annealing temperature of 62°C using primers as described by Orr et al1 Products were separated on 6% denaturing polyacrylamide gels and visualised by silver staining. The presence of a SfaNI restriction site was used to test for loss of CAT interruptions. Repetitive CAG sequences interrupted by at least one CAT triplet are digested by SfaNI. After restriction, PCR products were separated on a 2% agarose gel to assess the presence of a CAT interruption. Precise allele sizes and repeat configurations were determined by sequencing. To determine the nucleotide sequence of different alleles, PCR products were cloned into the vector pCR 2.1-TOPO (Invitrogen) and sequenced using the dideoxy chain termination method on double stranded DNA templates in presence of IRD800 labeled universal M13 primers on a Licor 4200 automated sequencer. For each template, up to ten single colonies have been analysed to detect the most representative repeat length. Due to somatic mosaicism in leukocytes, inaccuracy in amplification or sequencing reactions the repeat length may vary by ±1 unit between the subclones. Using the GeneScan methodology the number of repeats in a single allele varied usually between three and seven peaks on the scan.21
In addition, the instability of repetitive sequences in bacteria results in divergent subclones. Variability is frequent in expanded alleles as shown for the CAG50 allele in sample A but is the exception within the normal range.
Haplotype analysis
Microsatellite markers F13A1, D6S2434, D6S274, D6S288, D6S260, D6S285, D6S298, D6S2439, D6S1641 and D6S1017 closely linked to the SCA1 locus were selected for linkage analysis in two families. In pedigree I four living members are affected by SCA1. Three individuals of this family (L, M, N) could be examined. Pedigree II with seven individuals affected originating from the same geographigal region as pedigree I. Six patients could be examined presenting characteristic SCA1 features such as pale discs, intention tremor, pyramidal signs (data not shown in detail). Genotypes were determined by PCR amplification (35 cycles: 95°C 30 s, 58°C 30 s, 72°C 30 s). PCR products were resolved on 6% polyacrylamide gels and visualised by silver staining.
Results
The SCA1 repeat sizes and compositions in DNA samples of alleles larger than 35 triplets in the glutamine coding region were determined by PCR, restriction and sequence analyses. Interestingly, using silver staining after polyacrylamide gel electrophoresis in our hands the pattern of interrupted repeats may be differentiated from expanded alleles of identical length with pure CAG sequences (Figure 1). We suppose that the higher number of visible signals for pure repeat expansions is correlated with loss of CAT interruptions and may be produced either in consequence of mitotic instability or by the PCR reaction itself. Presence of CAT triplets within the CAG repeat was verified by SfaNI digestion.
PCR analysis of the SCA1 CAG repeat of normal and expanded alleles. In the upper part, PCR products were resolved on a denaturing polyacrylamide gel and detected by silver staining. Numbers of triplets as determined by sequencing are given. In the lower part, the agarose gel analysis before (−) and after SfaNI digestion (+) of the PCR products is shown. Expanded alleles with loss of CAT interruptions lack a SfaNI recognition site (marked by white arrows). SCA1 control samples: A, P.
Sequence analysis revealed that the 14 normal SCA1 alleles with 29 and 30 triplets as well as the alleles with 35–38 triplets are interrupted by the sequence CAT CAG CAT. In alleles with 39–41 triplets the interruption is missing in six of seven cases. DNA sequences are summarised in Table 1.
Alleles with 35–38 triplets are present in patients with ataxia but without additional characteristic features of SCA1 (B, C, D, F, G, H, I). SCA1 phenotypes were found for individuals Q and R with (CAG)41. For clinical data see Table 1.
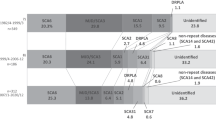
We found the allele with 39 triplets to be either formed by an interrupted or a pure CAG stretch. The perfect glutamine domain causes symptoms of SCA1 in family I (patients L+M+N) as well as in individual O. To investigate the genetic background of the 39 repeat allele in family I we performed haplotype analyses. We compared the allele distribution of 10 polymorphic markers tightly linked to the SCA1 locus between family I and a second pedigree (family II) originating from the same geographical region. The six affected persons of family II carry SCA1 alleles with repeat lengths between 48 and 51 CAG copies without interruptions as determined by restriction analyses. We found a common haplotype for the expanded SCA1 allele (marker distance between F13A1 and D6S285 about 16 cM) differing from normal alleles (Figure 2) indicating a common founder for both kindreds.
Characteristic features of SCA1 such as dysphagia, pale discs, intention tremor and pyramidal signs were present in the four SCA1 individuals with short expansions (mean age was 71.0±13.1 years). Their mean age of onset was 62.8±15.5 years with a mean disease duration of 8.3±3.2 years.
In the patient sample studied, the interrupted allele with 39 triplets did not cause characteristic features of SCA1: Individual K presented with prominent extrapyramidal symptoms such as choreoathetosis and dystonia and a very early disease onset. Basal ganglia dysfunction is rather uncommon in SCA1.27 In addition, early disease onset is generally thought to be associated with higher repeat numbers. Therefore, it is very unlikely that neurological symptoms in individual K are related to the interrupted 39 triplet allele.
Discussion
Here, we present the first familial case of stable inheritance of (CAG)39 SCA1 alleles missing CAT interruptions and correlating with symptoms characteristic for SCA1. Recently, Goldfarb et al21 found the (CAG)39 allele in affected family members of a large Siberian kindred (1484 individuals) but did not report stability of this SCA1 allele during transmission.
Our results give further evidence that the pathogenic development in SCA1 is caused by pure glutamine repeats without histidine residues that may change or stabilise the native protein.
The analysis of SCA1 alleles in individuals with 36–41 triplets suggests a change from normal to pathological alleles at 39 trinucleotides depending on the presence of CAT interruptions in the SCA1 repeat. These interruptions seem to modify the phenotype in critical alleles. Normal alleles with 39 repeats have been described in a Spanish kindred19 and are correlated with somatic stability for CAG sequences interrupted by a CAT trinucleotide.28
The clinical data in individuals with 39 triplets in the SCA1 repeat indicate that a perfect glutamine domain is necessary for the development of the SCA1 phenotype resulting in an altered function or processing of ataxin 1 at least in this intermediate range. Interruption of the domain by two histidine residues prevents or renders the aggregation or pathological effects more difficult. This hypothesis is also corroborated by a patient with an interrupted repeat of 58 triplets and a late onset at 50 years.23
Contrasting findings are described by Kennedy et al29 who reported a 39 triplet allele with CAT CAG CAT interruption in mother and son in one family with early age of onset. The brother of the female, who carries the same allele, is still asymptomatic at the age of 42. In this family, the development of pathological features is likely to be contingent upon additional factors.
Variability in disease expression or influence of unknown factors have also been reported in a second family30 where the 44 triplet SCA1 allele is interrupted twice by CAT CAG CAT. In contrast to the daughter who had mild developmental delay and truncal ataxia starting at the age of 2 years, her father carrying the identical allele failed to reveal signs of neurological impairment. In conclusion, the early and highly variable age at onset associated with interrupted sequences in the SCA1 gene is more likely to be caused by additional factors or other mutations in unrelated genes than on the interrupted allele itself.
For the individuals described here, SCA1 alleles with interruptions and shorter than 40 triplets are not associated with specific SCA1 features. In contrast, disease alleles are characterised by loss of interruptions. The 39 triplet allele with a pure CAG repeat in family I may be an expansion prone sequence. CAG repeats without CAT interruptions may be unstable during transmission as seen in family II. The corresponding haplotype in the SCA1 region suggests a common ancestor for these two pedigrees.
Careful molecular genetic investigation of intermediate alleles is highly required to support the clinical diagnosis and prognosis as well as for genetic counselling. Furthermore, the risk of expansion during transmission is estimated rather low for interrupted CAG stretches, but is considerably higher for pure CAG sequences resulting in anticipation in the next generation. Therefore, it is not sufficient to measure the repeat lengths in the intermediate range. The test for presence of CAT triplets within the CAG repeat should be included in molecular genetic diagnostics.
References
Orr HT, Chung M, Banfi S et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1 Nat Genet 1993 4: 221–226
Imbert G, Saudou F, Yvert G et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats Nat Genet 1996 14: 285–291
Pulst SM, Nechiporuk A, Nechiporuk T et al. Moderate expansion of a normally biallelic trinucleotide repeat in spino- cerebellar ataxia type 2 Nat Genet 1996 14: 269–276
Sanpei K, Takano H, Igarashi S et al. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT Nat Genet 1996 14: 277–284
Kawaguchi Y, Okamoto T, Taniwaki M et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1 Nat Genet 1994 8: 221–228
Zhuchenko O, Bailey J, Bonnen P et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the α-voltage-dependent calcium channel Nat Genet 1997 15: 62–69
David G, Abbas N, Stevanin G et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion Nat Genet 1997 17: 65–70
Koob MD, Moseley ML, Schut LJ et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8) Nat Genet 1999 1: 379–384
Matsuura T, Yamagata T, Burgess DL et al. Large expansion of the ATTCT pentanucleotide repeat in spinocerebellar ataxia type 10 Nat Genet 2000 26: 191–194
Holmes SE, O'Hearn EE, McInnis MG et al. Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is associated with SCA12 Nat Genet 1999 23: 391–392
Koide R, Ikeuchi T, Onodera O et al. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA) Nat Genet 1994 6: 9–13
Nagafuchi S, Yanagisawa H, Sato K et al. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p Nat Genet 1994 6: 14–18
Koide R, Kobayashi S, Shimohata T et al. A neurological disease caused by an expanded CAG trinucleotide repeat in the TATA-binding protein gene: a new polyglutamine disease? Hum Mol Genet 1999 8: 2047–2053
Zühlke Ch, Hellenbroich Y, Dalski A et al. Different types of repeat expansion in the TATA-binding protein gene are associated with a new form of inherited ataxia Eur J Hum Genet 2001 9: 160–164
Nakamura K, Jeong S-Y, Uchihara T et al. SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein Hum Mol Genet 2001 10: 1441–1448
Cummings CJ, Zoghbi HY . Forteen and counting: unraveling trinucleotide repeat diseases Hum Mol Genet 2000 9: 909–916
Bürk K, Bösch S, Globas C et al. Cognitive deficits in Spinocerebellar Ataxia 1 (SCA1) Eur Neurol 2001 46: 43–48
Kish SJ, el Awar M, Stuss D et al. Neuropsychological test performance in patients with dominantly inherited spinocerebellar ataxia: relationship to ataxia severity Neurology 1994 44: 1738–1746
Matilla T, Volpini V, Genis D et al. Presymptomatic analysis of spinocerebellar ataxia type 1 (SCA1) via the expression of the SCA1 CAG-repeat in a large pedigree displaying anticipation and parental male bias Hum Mol Genet 1993 2: 2123–2128
Banfi S, Servadio A, Chung M et al. Identification and characterization of the gene causing type 1 spinocerebellar ataxia Nat Genet 1994 7: 513–520
Goldfarb LG, Vasconcelos O, Platonov FA et al. Unstable repeat and phenotypic variability of spinocerellar ataxia type 1 Ann Neurol 1996 39: 500–506
Chung M, Ranum LPW, Duvick L, Servadio A, Zoghbi HY, Orr HT . Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type 1 Nat Genet 1993 5: 254–258
Matsuyama Z, Izumi Y, Kameyama M, Kawakami H, Nakamura S . The effect of CAT trinucleotide interruptions on the age at onset of spinocerebellar ataxia type 1 (SCA1) J Med Genet 1999 36: 546–548
Rubinsztein DC, Leggo J, Coles R et al. Phenotypic characterization of individuals with 30 - 40 repeats in the Huntington Disease gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36–39 repeats Am J Hum Genet 1996 59: 16–22
Andrew SE, Goldberg YP, Hayden MR . Rethinking genotype and phenotype correlations in polyglutamine expansion disorders Hum Mol Genet 1997 6: 2005–2010
Miller SA, Dykes DD, Polesky HF . A simple salting out procedure for extracting DNA from human nucleated cells Nucl Acids Res 1988 16: 1215
Bürk K, Abele M, Fetter M et al. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3 Brain 1996 119: 1497–1505
Chong S, McCall AE, Cota J et al. Gametic and somatic tissue-specific heterogeneity of the expanded SCA1 CAG repeat in spinocerebellar ataxia type 1 Nat Genet 1995 10: 344–350
Kennedy D, Skuterud M, Florio P, Orr HT, Wyatt P . Unexpected findings from a Canadian diagnostic laboratory conducting molecular testing for spinocerellar ataxia type 1 Am J Hum Genet 1996 59 suppl: A226
Quan F, Janas J, Popovich BW . A novel CAG repeat configuration in the SCA1 gene: implications for the molecular diagnostics of spinocerellar ataxia type 1 Hum Mol Genet 1995 4: 2411–2413
Acknowledgements
We would like to thank J Atici and U Gehlken for excellent technical help. This work was supported by the Forschungsförderungsprogramm der Medizinischen Universität Lübeck (1799/N03) and the Fritz Thyssen Stiftung, Köln (AZ 1999 2060). We thank all patients for providing blood samples for scientific research and their clinicians for collecting them. We thank the German Heredo-Ataxia Society (DHAG), whose cooperation is essential in our work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zühlke, C., Dalski, A., Hellenbroich, Y. et al. Spinocerebellar ataxia type 1 (SCA1): Phenotype-genotype correlation studies in intermediate alleles. Eur J Hum Genet 10, 204–209 (2002). https://doi.org/10.1038/sj.ejhg.5200788
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5200788
Keywords
This article is cited by
-
Essential Tremor Within the Broader Context of Other Forms of Cerebellar Degeneration
The Cerebellum (2020)
-
Genetics, Mechanisms, and Therapeutic Progress in Polyglutamine Spinocerebellar Ataxias
Neurotherapeutics (2019)
-
Expansion, mosaicism and interruption: mechanisms of the CAG repeat mutation in spinocerebellar ataxia type 1
Cerebellum & Ataxias (2016)
-
Experience and outcome of 3 years of a European EQA scheme for genetic testing of the spinocerebellar ataxias
European Journal of Human Genetics (2008)
-
Direct and accurate measurement of CAG repeat configuration in the ataxin-1 (ATXN-1) gene by “dual-fluorescence labeled PCR-restriction fragment length analysis”
Journal of Human Genetics (2008)