Abstract
Background/Objectives:
It is unknown whether short sleep duration causatively contributes to weight gain. Studies investigating effects of partial sleep deprivation (PSD) on energy balance components report conflicting findings. Our objective was to conduct a systematic review and meta-analysis of human intervention studies assessing the effects of PSD on energy intake (EI) and energy expenditure (EE).
Subjects/Methods:
EMBASE, Medline, Cochrane CENTRAL, Web of Science and Scopus were searched. Differences in EI and total EE following PSD compared with a control condition were generated using the inverse variance method with random-effects models. Secondary outcomes included macronutrient distribution and resting metabolic rate. Heterogeneity was quantified with the I2-statistic.
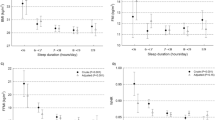
Results:
Seventeen studies (n=496) were eligible for inclusion in the systematic review, and 11 studies (n=172) provided sufficient data to be included in meta-analyses. EI was significantly increased by 385 kcal (95% confidence interval: 252, 517; P<0.00001) following PSD compared with the control condition. We found no significant change in total EE or resting metabolic rate as a result of PSD. The observed increase in EI was accompanied by significantly higher fat and lower protein intakes, but no effect on carbohydrate intake.
Conclusions:
The pooled effects of the studies with extractable data indicated that PSD resulted in increased EI with no effect on EE, leading to a net positive energy balance, which in the long term may contribute to weight gain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015; 1: 40–43.
Matricciani L, Olds T, Petkov J . In search of lost sleep: Secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev 2012; 16: 203–211.
Bin YS, Marshall NS, Glozier N . Secular trends in adult sleep duration: a systematic review. Sleep Med Rev 2012; 16: 223–230.
Bin YS, Marshall NS, Glozier N . Sleeping at the limits: The changing prevalence of short and long sleep durations in 10 countries. Am J Epidemiol 2013; 177: 826–833.
Boivin DB, Boudreau P . Impacts of shift work on sleep and circadian rhythms. Pathol Biol 2014; 62: 292–301.
Krueger PM, Friedman EM . Sleep duration in the united states: a cross-sectional population-based study. Am J Epidemiol 2009; 169: 1052–1063.
Shochat T . Impact of lifestyle and technology developments on sleep. Nat Sci Sleep 2012; 4: 19–31.
Ikehara S, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep 2009; 32: 295–301.
Moreno CRC, Louzada FM, Teixeira LR, Borges F, Lorenzi-Filho G . Short sleep is associated with obesity among truck drivers. Chronobiol Int 2006; 23: 1295–1303.
Cappuccio FP, Taggart FM, Kandala N-B, Currie A, Peile E, Stranges S et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008; 31: 619–626.
Patel SR, Hu FB . Short sleep duration and weight gain: a systematic review. Obesity 2008; 16: 643–653.
Cappuccio F, D’Elia L, Strazzullo P, Miller M . Quantity and quality of sleep and incidence of type 2 diabetes a systematic review and meta-analysis. Diabetes Care 2010; 33: 414–420.
Cappuccio FP, Cooper D, Delia L, Strazzullo P, Miller M . Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011; 32: 1484–1492.
Wang Q, Xi B, Liu M, Zhang Y, Fu M . Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res 2012; 35: 1012–1018.
Magee L, Hale L . Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep Med Rev 2012; 16: 231–241.
Marshall NS, Glozier N, Grunstein RR . Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev 2008; 12: 289–298.
Capers PL, Fobian AD, Kaiser K, Borah R, Allison DB . A systemic review and meta-analysis of randomized controlled trials of the impact of sleep duration on adiposity and components of energy balance. Obes Rev 2015; 16: 771–782.
Higgins J, Green S . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. The Cochrane Collaboration, 2011, Available from http://handbook.cochrane.org/.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Ioannidis JP, Clarke M et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions. Ann Intern Med 2009; 15: W65–W94.
Higgins JPT, Altman DG SJ . Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011, Available from http://handbook.cochrane.org/.
Higgins J, Thompson S . Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558.
Egger M, Smith G, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts 2008; 1: 266–273.
St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011; 94: 410–416.
Brondel L, Romer M a, Nougues PM, Touyarou P, Davenne D . Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010; 91: 1550–1559.
Calvin AD, Carter RE, Adachi T, MacEdo PG, Albuquerque FN, Van Der Walt C et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 2013; 144: 79–86.
Hart CN, Carskadon MA, Demos KE, Van Reen E, Sharkey KM, Raynor HA et al. Acute changes in sleep duration on eating behaviors and appetite-regulating hormones in overweight/obese adults. Behav Sleep Med 2014; 13: 424–436.
Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA 2013; 110: 5695–5700.
Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD . Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009; 89: 126–133.
Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr 2009; 90: 1476–1482.
Shechter A, Rising R, Wolfe S, Albu JB, St-Onge MP . Postprandial thermogenesis and substrate oxidation are unaffected by sleep restriction. Int J Obes 2013; 38: 1153–1158.
Shechter A, Rising R, Albu JB, St-Onge MP . Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr 2013; 98: 1433–1439.
Spaeth AM, Dinges DF, Goel N . Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 2013; 36: 981–990.
Spaeth AM, Dinges DF, Goel N . Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr 2014; 100: 559–566.
Brondel L, Romer MA, Nougues P, Touyarou P, Davenne D . Acute partial sleep deprivation increases food intake in healthy young men. Fund Clin Pharm 2011; 25: 26 (abstract).
Calvin AD, Carter RE, Levine JA, Somers VK . Insufficient sleep increases caloric intake but not energy expenditure. Circulation 2012; 125: S1 (abstract).
Markwald RR, Melanson EL, Smith MR, Perreault L, Eckel RH, Wright KP . Effect of sleep restriction on whole body energy expenditure in humans. Sleep 2011; 34: A41 (abstract).
Roberts AL, Kelleman M, St-Onge M-P . Short sleep duration tends to lower resting metabolic rate and peak activity level relative to habitual sleep in normal weight subjects. Obesity 2010; 18: S83 (abstract).
Shechter A, O’Keefe M, Roberts AL, Zammit G, RoyChoudhury A, St-Onge M-P . Altered nocturnal sleep architecture in response to partial sleep deprivation is associated with increased carbohydrate intake. Sleep 2012; 35: A104 (abstract).
Spaeth AM, Dinges DF, Goel N . Sleep restriction associates with increased caloric intake and delayed meal timing in healthy adults. Sleep 2013; 36: A106 (abstract).
Spaeth AM, Goel N, Dinges D . Baseline slow-wave sleep negatively relates to energy balance responses during leep restriction in healthy adults. Sleep 2014; 37: A50 (abstract).
Spaeth AM, Goel N, Dinges DF . Sleep restriction associates with increased food intake, weight gain and changes in food cravings in healthy adults. Sleep 2012; 35: A105 (abstract).
Spaeth AM, Goel N, Dinges DF . Effects of sleep restriction on body weight and food intake in healthy adults. Appetite 2012; 59: e51 (abstract).
Spaeth AM, Goel N, Dinges DF . Effects of two five-day bouts of chronic sleep restriction on caloric intake in healthy adults. Sleep 2013; 36: A87 (abstract).
Spaeth AM, Wohl R, Dinges DF, Goel N . Stability of energy balance responses to sleep restriction over long time intervals. Sleep 2014; 37: A47 (abstract).
Demos KE, Carskadon MA, Sharkey KM, Hart CN, Cairns A, Lawton JM, Ogilvie R, Wing RR . Effects of acute changes in scheduled sleep duration on eating behavior. Obesity 2011; 19: S194 (abstract).
Kubota C, Hibi M, Mizuno T, Mitsui Y, Uchida S . Effect of sleep restriction on physical functions—a respiratory chamber study. Sleep 2013; 36: A105 (abstract).
LeCheminant JD, Romney L, Clark T, Bailey BW, Larson M, Tucker LA . The relationship between sleep deprivation and the energy balance pathways of diet and physical activity. Med Sci Sports Exerc 2013; 45: 336 (abstract).
Tasali E, Broussard J, Day A, Kilkus J, Van Cauter E . Sleep curtailement in healthy young adults is associated with increased ad lib food intake. Sleep 2009; 32: A131 (abstract).
Taylor MK, Gibson CA, Unruh GK, Ptomey LT, Spaeth KR, Sullivan DK, Dietary intake of sleep-deprived, on-call anesthesiology residents. FASEB J 2013; 27: 1064–1 (abstract).
Bromley LE, Booth JN, Kilkus JM, Imperial JG, Penev PD . Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep 2012; 35: 977–984.
St-Onge M-P . The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med 2013; 9: 73–80.
Shepard JW, Buysse DJ, Chesson AL, Dement WC, Goldberg R, Guilleminault C et al. History of the development of sleep medicine in the United States. J Clin Sleep Med 2005; 1: 61–82.
Spiegel K, Tasali E, Penev P, Van Cauter E . Article Brief Communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141: 846–851.
St-Onge M-P, Mcreynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J . Sleep restriction leads to increased activation of brain regions sensitive. Am J Clin Nutr 2012; 95: 818–824.
Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C . From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite 2007; 48: 12–19.
Mozaffarian D, Micha R, Wallace S . Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010; 7: 1–10.
Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira M, Balter K, Fraser GE et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009; 89: 1425–1433.
Schwingshackl L, Strasser B, Hoffmann G . Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab 2011; 59: 176–186.
Klingenberg L, Chaput J, Holmback U, Jennum P, Astrup A, Sjodin A . Sleep restriction is not associated with a positive energy balance in adolescent boys. Am J Clin Nutr 2012; 96: 240–248.
Garby L, Kurzer M, Lammert O, Nielsen E . Energy expenditure durign sleep in men and women: evaporative and sensible heat losses. Hum Nutr Clin Nutr 1987; 41: 225–233.
Goldberg G, Prentice A, Davies HL, Murgatroyd P . Overnight and basal metabolic rates in men and women. Eur J Clin Nutr 1988; 42: 137–144.
Seale J, Conway J . Relationship between overnight energy expenditure and BMR measured in a room-sized calorimeter. Eur J Clin Nutr 1999; 53: 107–111.
Bonnet MH, Arand DL . 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. Sleep NY 1995; 18: 581–588.
Shechter A, Varin F, Boivin DB . Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep 2010; 33: 647–656.
Dalvit SP . The effect of the menstrual cycle on patterns of food intake. Am J Clin Nutr 1981; 34: 1811–1815.
Webb P . 24-Hour energy expenditure menstrual. Am J Clin Nutr 1986; 44: 614–619.
Bisdee JT, James WP, Shaw MA . Changes in energy expenditure during the menstrual cycle. Br J Nutr 1989; 61: 187–199.
Piers LS, Diggavi SN, Rijskamp J, Van Raaij JM a, Shetty PS, Hautvast JG . Resting metabolic rate and thermic effect of a meal in the follicular and luteal phases of the menstrual cycle in well-nourished Indian women. Am J Clin Nutr 1995; 61: 296–302.
Calvin AD, Carter RE, Levine JA, Somers VK . Insufficient sleep increases caloric intake but not energy expenditure. Circulation 2012; 1 (abstract).
Shlisky JD, Hartman TJ, Kris-Etherton PM, Rogers CJ, Sharkey NA, Nickols-Richardson SM . Partial sleep deprivation and energy balance in adults: an emerging issue for consideration by dietetics practitioners. J Acad Nutr Diet 2012; 112: 1785–1797.
Acknowledgements
The authors’ responsibilities were as follows: GP and JD designed the study, HA, SH, JD, and GP performed the literature search and the meta-analysis. GP and JD had primary responsibility for final content. All authors were substantially involved in the writing process. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Al Khatib, H., Harding, S., Darzi, J. et al. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur J Clin Nutr 71, 614–624 (2017). https://doi.org/10.1038/ejcn.2016.201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2016.201
This article is cited by
-
Dietary taste patterns and diet quality of female nurses around the night shift
European Journal of Nutrition (2024)
-
Shorter sleep among adolescents is associated with lower fruit and vegetable consumption the following day
International Journal of Behavioral Nutrition and Physical Activity (2023)
-
Sleep Technology Intervention to Target Cardiometabolic Health (STITCH): a randomized controlled study of a behavioral sleep extension intervention compared to an education control to improve sleep duration, blood pressure, and cardiometabolic health among adults with elevated blood pressure/hypertension
Trials (2023)
-
The role of insufficient sleep and circadian misalignment in obesity
Nature Reviews Endocrinology (2023)
-
Randomized controlled trial to enhance children’s sleep, eating, and weight
Pediatric Research (2022)