Abstract
Objectives:
To study the effect of Vitamin D3 supplementation on metabolic control in an obese type 2 diabetes Emirati population.
Methods:
This randomized double-blind clinical trial was conducted with 87 vitamin D-deficient obese, type 2 diabetic participants. The vitamin D-group (n=45) and the placebo group (n=42) were matched for gender, age, HbA1c and 25-hydroxy vitamin D (25(OH) D) at the baseline. The study was divided into two phases of 3 months each; in phase 1, the vitamin D-group received 6000 IU vitamin D3/day followed by 3000 IU vitamin D3/day in phase 2, whereas the placebo group (n=42) received matching placebo.
Results:
After supplementation, serum 25(OH) D peaked in the vitamin D-group in phase 1 (77.2±30.1 nmol/l, P=0.003) followed by a decrease in the phase 2 (61.4±18.8 nmol/l, P=0.006), although this was higher compared with baseline. In the placebo group, no difference was observed in the serum 25(OH) D levels throughout the intervention. Relative to baseline serum, parathyroid hormone decreased 24% (P=0.003) in the vitamin D-group in phase 2, but remained unchanged in the placebo group. No significant changes were observed in blood pressure, fasting blood glucose, HbA1c, C-peptide, creatinine, phosphorous, alkaline phosphatase, lipids, C-reactive protein or thyroid stimulating hormone concentrations compared with baseline in either group.
Conclusions:
Six months of vitamin D3 supplementation to vitamin D-deficient obese type 2 diabetes patients in the UAE normalized the vitamin D status and reduced the incidence of eucalcemic parathyroid hormone elevation but showed no effect on the metabolic control.
Similar content being viewed by others
Introduction
Vitamin D role in calcium homeostasis and bone metabolism is well established.1 However, in recent years, there has been numerous non-skeletal disease associations reported with vitamin D deficiency including type 2 diabetes mellitus (T2D). This additional evidence for the role of vitamin D in T2D comes from a number of epidemiological studies showing an inverse association between vitamin D status and hyperglycemia,2, 3 insulin resistance4 prevalence of T2D5, 6 and obesity.7
The prevalence of diabetes mellitus in the United Arab Emirates (UAE) has been reported to be ~20% in 2013 by the International Diabetes Federation,8 and this has been attributed to the rapid economic development coupled with ageing populations. This rapid urbanization, economic growth and technological advances have also contributed to the alarming rise of obesity in this region.9
Vitamin D deficiency is startlingly high in the UAE and other Gulf Cooperation Council countries, where the intensity and hours of sunlight is paradoxically proportional to the prevalence of vitamin D deficiency.10, 11
The demonstration of a causal relation between vitamin D and glucose metabolism needs evidence from randomized adequately powered placebo-controlled intervention trials. As reviewed recently, the studies published so far on T2D subjects have a number of methodological limitations and shortcomings, such as small sample sizes, heterogeneous regarding dose and formulation of vitamin D treatment, duration, inclusion–exclusion criteria and study populations. However, these studies have often yielded inconclusive results.12, 13
In the UAE, no randomized controlled trial has been reported in vitamin D-deficient obese T2D patients. The appropriate dosing regimen for improving vitamin D status in this population is insufficiently studied. The purpose of this study was to conduct a double-blind randomized trial in the Emirati population to clarify the relationship between vitamin D3 (cholecalciferol) supplementation and glycemic control. Also in our study, we aimed to give large enough doses of vitamin D3 to normalize (>50 nmol/l) serum 25-hydroxy vitamin D (25(OH) D).
Methodology
Participants
This trial was conducted between June 2012 and December 2012 at Rashid Center for Diabetes and Research, a tertiary diabetes care facility in Ajman, UAE. The criteria for eligibility were UAE nationality, male and female, age between 30 and 60 years, body mass index (BMI)⩾30 kg/m2, diagnosis of T2D and serum 25(OH) D<50 nmol/l. The participants were excluded if they were diagnosed with type 1 diabetes, taking vitamin D supplementation, serum creatinine >200 μmol/l, liver function tests (alkaline phosphates, alanine amino transferase and bilirubin) greater than three times the upper limit of the local reference range, possible primary hyperparathyroidism (plasma parathyroid hormone (PTH) >5.0 pmol/l combined with serum calcium >2.8 mmol/l), malabsorption syndrome, on hormone replacement therapy, corticosteroids or anti-convulsants. Pregnant or lactating women were also excluded.
Study design
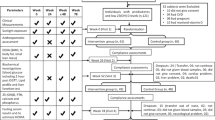
The summary of the study design is illustrated in Figure 1. The recruited participants were randomized by a computer-generated random number sequence into one of the two groups—the vitamin D-group (D-group) and the placebo group (P-group)—with stratification according to age, gender and BMI by the Rashid Center for Diabetes and Research, Information Technology department. The participants and the research team remained blinded to treatment allocation until after the final analysis was completed. Vitamin D3 (Solgar, Leonia, NJ, USA) and placebo (starch) (Compound Pharmacy, Dubai, UAE) were in capsule form and identical in appearance. They were pre-packed in bottles and consecutively numbered to be dispensed by an independent pharmacist according to the randomization list.
The study was divided into two phases of 3 months each. In phase 1, the D-group (n=45) received unlabelled oral vitamin D3 6000 IU/day, whereas the P-group (n=42) received placebo capsules. In phase 2, the D-group (n=45) received 3000 IU/day of oral vitamin D3 and the P-group (n=42) continued with matching placebo capsules (Figure 1). Participants were advised to maintain their usual medical care and diets and avoid taking calcium or vitamin D supplements on their own while in the study period. They were also informed to report any interval morbidity including nausea, vomiting, excessive thirst, frequent urination, constipation, abdominal pain, weakness and confusion. A study staff contacted the participants by phone after 3 and 6 weeks of initiation of the trial to ensure that the study medication was taken correctly and to register any adverse events. Compliance was recorded in a questionnaire at 3 and 6 months and was assessed by measuring 25(OH) D concentrations after un-blinding.
Of the 366 patients who were screened for eligibility by the physician, 279 were excluded and 87 (23.8%) were recruited after a written informed consent was undertaken by the study staff. This study was conducted according to the guidelines laid down by the Declaration of Helsinki and all procedures involving the participants were approved by the Ethical committee of the Ministry of Health, UAE.
Measurements
A standard pretested questionnaire was completed by the participants to record information about outdoor sun exposure, use of sunscreen, clothing and skin colour (Fitzpatrick scale).14 Systolic and diastolic blood pressure was measured after 5–10 min rest, on the right arm in the sitting position. Fasting (12 h) venous blood sample (8 ml) was collected and assessed for fasting blood glucose, HbA1c, C-peptide, PTH, lipid profile (cholesterol, triglyceride, high-density lipoprotein-cholesterol and low-density lipoprotein-cholesterol), apolipoprotein A, Apolipoprotein B, phosphorous, calcium, C-reactive protein, creatinine, alkaline phosphatase and thyroid stimulating hormone in a Roche COBAS 6000 analyzer (Mannheim, Germany). Blood HbA1c was reported in the NGSP (%) unit, which is used in UAE. These results can be translated to SI (IFCC) units using the International Federation of Clinical Chemistry (IFCC)-National Glycohemoglobin Standardization Program (NGSP) ‘master equation’ HbA1c (NGSP)=0.9148 × HbA1c (IFCC)+2.152.15 25(OH) D was measured using immunochemiluminescene, performed in Diasorin Liaison analyzer (Saluggia, Italy). The analyzing laboratory participated in the vitamin D external quality assessment scheme. The lab tests were repeated at the baseline end of phases 1 and 2.
Statistical analysis
The study was powered to detect 0.75% difference in HbA1c, 0.7 kg/m2 in BMI and 6 mm Hg in blood pressure between the groups. The estimated sample size of 50 subjects in each group would give a power of 90% with 95% confidence interval. Statistical evaluations were performed using SPSS 13.0 (IBM Corp, NY, USA. Released 2013). The biochemical variables that were non-normally distributed were normalized by logarithmic transformation. Comparison between the groups at baseline was done using Student's t-test and between baseline and phases 1 and 2 were done by repeated-measures analysis of variance in both the groups. The Post hoc least-square difference was performed to identify the significant group for each significant variable on the analysis of variance test. All statistical tests were performed two-sided, and P-value⩽0.05 was considered statistically significant. No data were attributed for missing data and analyzed for intent to treat. A secondary per-protocol analysis was also conducted for the accuracy of primary analysis and yielded results in similar significance and direction (not presented).
Results
Eighty-seven participants were randomly assigned into two groups—the D-group (n=45) and the P-group (n=42); there was no drop out reported at the end of phase 1 (3 months); in phase 2 (3 months) we lost three subjects in each arm as shown in Figure 1. At the end of the trial, 82 subjects—that is, D-group (n=43) and P-group (n=39)—were analyzed.
The baseline characteristics of the participants are presented in Table 1, and there was no significant difference (P<0.05) in age, BMI, serum 25(OH) D levels, HbA1c or duration of diabetes between randomly allocated groups. The oral anti-diabetic drugs included biguanides, thiazolidinediones, sulphonylureas and incretins. All participants wore traditional attire—kandoora (male) or Abaya (female)—when outdoor, which limits surface area exposure to direct sunlight. Less than 5 min/day exposure to direct sunlight was reported by 33 and 44% of participants in D and P-group. The average temperatures in the UAE were between 30 and 47 °C (maximum) and 17 and 27 °C (minimum) during the study period (June to December 2012).The Fitzpatrick scale assessed 84% of the participants between grade IV and V—that is, between minimally sun sensitive to sun insensitive, burns minimally to rarely burns and always tans to moderate brown to tans well.
The Pearson correlation coefficient (r) at the baseline (n=87) showed an inverse relationship between serum 25(OH) D and fasting blood glucose (r=−0.27, P<0.01), HbA1c (r=−0.25, P<0.05), total cholesterol (r=−0.23, P<0.05) and triglycerides (r=−0.30, P<0.01).
The effect of vitamin D3 supplementation is shown in Table 2. Serum 25(OH) D increased (P=0.003) by the end of phase 1 followed by a decrease (P<0.01) in phase 2 but maintained a higher level compared with the baseline value (P<0.01), whereas serum PTH decreased in phase 2 (P=0.003) compared with the baseline. Concurrently, 25(OH) D and calcium decreased (P<0.05) after 6 months in the P-group.
Supplementation of vitamin D3 did not show statistical difference in glycemic control (fasting blood glucose, HbA1c and C-peptide), lipids, C-reactive protein, creatinine, (Table 2), apolipoproteins A and B and thyroid stimulating hormone (not shown in the table).
Sub analysis:
The observation remained consistent even after stratifying the groups according to BMI and serum 25(OH) D. The D-group was divided into two subgroups—that is, HbA1C⩽8% (n=22) and HbA1c >8% (n=23)—and compared baseline with phase 2, and none of the variables showed any statistical difference, except for an increase in 25(OH) D (P<0.001) in both the subgroups but a decrease (P<0.05) in PTH only in the group with HbA1C⩽8%.
The effect of vitamin D supplementation on a subgroup with serum 25(OH) D <30 nmol/l showed no improvement in any of the metabolic markers. The group (n=24) that achieved serum 25(OH) D ⩾75 nmol/l in phase 1 versus the placebo showed no changes in the metabolic markers either.
Discussion
This 6-month randomized double-blind placebo-controlled study in vitamin D-deficient obese T2D Emirati patients showed no effect of vitamin D3 supplementation on glycemic control.
The subjects recorded a strikingly low exposure to direct sunlight, although the climate of the UAE is generally hot and dry with ample sunlight throughout the year; the soaring temperatures in the summer (June–September average temperature 42 °C) restrict outdoor activities. Similar observation has been reported earlier in this region.16
At baseline, serum 25(OH) D was inversely related to glycemia (fasting blood glucose and HbA1C). From a theoretical point of view, there are several mechanisms whereby vitamin D may influence glycemic control, insulin secretion and sensitivity. For instance, the vitamin D receptor is expressed in the insulin producing beta cells. These cells also have the enzyme (CYP27B1) necessary for activating 25(OH) D to the active form 1,25 dihydroxy vitamin D. Added to this, the insulin secretion is also a calcium-dependent process and can consequently be indirectly influenced by vitamin D, which is a known regulator of calcium balance. Moreover, vitamin D may also stimulate the expression of the insulin receptor in peripheral tissues and thereby increase glucose transport.17, 18, 19
Another observation in our study was that the baseline C-reactive protein levels were high (8.0±7.6 mg/l), which is above the standard reference range (<4 mg/l). This was higher than participants of white, Hispanic, African-American and Asian ethnicity.20 Only four patients had C-reactive protein below 1.0 mg/l, and 20 patients had C-reactive protein levels >10.0 mg/l. This may indicate a high-grade inflammation in centrally obese diabetic subjects in the UAE.
On supplementation, we found neither dose (6000 or 3000 IU/day) of vitamin D3 could statistically influence the glycemic control (HbA1c, fasting blood glucose and c-peptide) nor was there any change in other metabolic markers (Table 2). However, there was an increase (P<0.01) in the serum 25(OH) D and a decrease in PTH in the D-group and alongside a decrease (P<0.05) in serum 25(OH) D and calcium in the P-group compared with baseline.
The results from small clinical trials and post hoc analyses of larger trials on the effect of vitamin D supplementation on glycemic control have been inconsistent. A systematic review of nine studies suggested a weak effect of vitamin D supplementation in reducing fasting blood glucose and improving insulin resistance and no change in HbA1c in subjects with T2D and impaired glucose tolerance.21 Three small underpowered randomized controlled trials in T2D patients show no change in fasting blood glucose, HbA1c or insulin resistance after a follow-up period of 8–26 weeks. This controversy, or at least part of it, might be due to the ethnicity differences in the study’s population.22, 23, 24, 25
Detecting an increase in serum 25(OH) D in obese diabetic subjects following vitamin D supplementation is in agreement to all the supplementation studies.21 The significant (28.5±9.2 vs 77.2±30.1, P=0.003) but lower than anticipated rise in serum 25(OH) D may be because of ethnicity-related differences in vitamin D absorption and/or altered vitamin D metabolism in the obese subjects.26, 27
However, the supplementation dose was selected on the basis of the recommendations of Endocrine Society Clinical guidelines 2011,28 which suggested 6000–10 000 IU/day of vitamin D3 to treat vitamin D deficiency and maintain 25(OH)D level above 30 ng/ml (75 mmol/l), followed by maintenance therapy of 3000–6000 IU vitamin D3/d. Our findings show that 6000 IU vitamin D3/day for 3 months safely increased serum 25(OH) D, but was probably low to optimize all vitamin D-dependent functions. Some authors consider 75 nmol/l to be an adequate concentration to begin with, and the best to be between 90 and 100 nmol/l (36–40 ng/ml),29 although this is still controversial. Wortsman et al27 have reported that the obese participants were able to raise their blood levels of vitamin D by no more that 50% compared with the non-obese adults. Hence, we presume this could have also caused a suboptimal increase in the circulating 25(OH) D in our obese subjects.
The supplementation of vitamin D3 suppressed the PTH levels after phase 2 (5.9±2.4 vs 4.4±1.8), unlike in the P-group. which showed a small but insignificant increase in PTH. These results are in line with the fact that PTH decreases with increasing 25(OH) D concentrations as reported earlier.23 No significant change in blood pressure was recorded; this could be attributed to a well controlled baseline mean systolic and diastolic blood pressure. These results are supported by a meta-analysis where a small reduction in blood pressure was reported with vitamin D therapy but only in studies with a mean baseline systolic blood pressure of >140 mm Hg.24
In the present study, most of the patients were taking statins and angiotensin converting enzyme inhibitors at the baseline and the lipid profile was well controlled, and supplementation did not show any significant improvement. A meta-analysis of 12 clinical trials reported no significant effect of vitamin D supplementation on total cholesterol, high-density lipoprotein-cholesterol and triglyceride.30
To our knowledge, this is the first randomized double-blind placebo-controlled trial of vitamin D supplementation in obese T2D Emirati participants. In the current study, we have recorded a wide range of metabolic markers. There was a well-matched baseline group, and all the participants in the study were receiving medical care suggestive of current standards of care.31
The limitation of the study could be the inefficiency to raise and sustain the serum 25 (OH) D>75 nmol/l, attributing it to the compliance (85%) in both the groups, insufficiency of the dose of vitamin D3 and/or interaction with patient medication. However, in a recent study by Davidson et al32 a mean weekly dose of 89 000 IU in individuals with prediabetes and hypovitaminosis had no effect on the development of diabetes, insulin secretion or insulin sensitivity, although a slight reduction in HbA1c levels was observed. Therefore, we consider it unlikely that a higher dose of vitamin D or a better compliance would have changed the trial outcome significantly.
Conclusion
In conclusion, the present trial has indicated that, although there was an inverse baseline relationship between serum 25(OH) D and glycemic profile, the vitamin D3 supplementation in the obese T2D participants in the UAE did not improve glycaemic control or lipid concentrations. However, the treatment with vitamin D3 reduced the incidence of secondary hyperparathyroidism and normalized the PTH levels.
References
Scragg R . Do we need to take calcium with vitamin D supplements to prevent falls, fractures, and death? Curr Opin Clin Nutr Metab Care 2012; 15: 614–624.
Scragg R, Sowers M, Bell C . Third National Health and Nutrition Examination Survey Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004; 27: 2813–2818.
Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ . Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes 2008; 57: 2619–2625.
Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010; 33: 1379–1381.
Knekt P, Laaksonen M, Mattila C, Härkänen T, Marniemi J, Heliövaara M et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008; 19: 666–671.
Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jenssen T, Njølstad I et al. Baseline serum 25-hydroxyvitamin D concentrations in the Tromsø Study 1994-95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up. Diabet Med 2010; 27: 1107–1115.
Vanlint S . Vitamin D and obesity. Nutrients 2013; 5: 949–956.
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE . Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103: 137–149.
Mehio Sibai A, Nasreddine L, Mokdad AH, Adra N, Tabet M, Hwalla N . Nutrition transition and cardiovascular disease risk factors in Middle East and North Africa countries: reviewing the evidence. Ann Nutr Metab 2010; 57: 193–203.
Sadiya A, Ahmed SM, Skaria S, Abusnana S . Vitamin D status and its relationship with metabolic markers in persons with obesity and type 2 diabetes in the UAE: a cross-sectional study. J Diabetes Res 2014; 2014: 869307.
Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, El-Kholie E, Yousef M et al. Increased vitamin D supplementation recommended during summer season in the gulf region: a counterintuitive seasonal effect in vitamin D levels in adult, overweight and obese Middle Eastern residents. Clin Endocrinol (Oxf) 2012; 76: 346–350.
Mitri J, Muraru MD, Pittas AG . Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr 2011; 65: 1005–1015.
Griz LH, Bandeira F, Gabbay MA, Dib SA, Carvalho EF . Vitamin D and diabetes mellitus: an update 2013. Arq Bras Endocrinol Metabol 2014; 58: 1–8.
Fitzpatrick TB . Ultraviolet-induced pigmentary changes: benefits and hazards. Curr Probl Dermatol 1986; 15: 25–38.
Hoelzel W, Weykamp C, Jeppsson JO, Miedema K, Barr JR, Goodall I et al. IFCC Working Group on HbA1c Standardization. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem 2004; 50: 166–174.
Al Anouti F, Thomas J, Abdel-Wareth L, Rajah J, Grant WB, Haq A . Vitamin D deficiency and sun avoidance among university students at Abu Dhabi, United Arab Emirates. Dermatoendocrinol 2011; 3: 235–239.
Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol 2004; 89-90: 121–125.
Maestro B, Campión J, Dávila N, Calle C . Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 2000; 47: 383–391.
Baynes KC, Boucher BJ, Feskens EJ, Kromhout D . Vitamin D, glucose tolerance and insulinaemia in elderly men. Diabetologia 1997; 40: 344–347.
Albert MA, Glynn RJ, Buring J, Ridker PM . C-reactive protein levels among women of various ethnic groups living in the United States (from the Women's Health Study). Am J Cardiol 2004; 93: 1238–1242.
George PS, Pearson ER, Witham MD . Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med 2012; 29: e142–e150.
Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD . Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med 2008; 25: 320–325.
Jorde R, Sneve M, Torjesen P, Figenschau Y . No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med 2010; 267: 462–472.
Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD . The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 2010; 53: 2112–2119.
Chiu KC, Chu A, Go VL, Saad MF . Hypovitaminosis D is associated with insulin resistance and beta cell function. Am J Clin Nutr 2004; 79: 820–825.
Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 2008; 87: 1952–1958.
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF . Decreased bioavailability of vitamin D in obesity. Erratum in: Am J Clin Nutr. 2003; 77(5):1342. Am J Clin Nutr 2000; 72: 690–693.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930.
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Review. [Published erratum appears in Am J Clin Nutr 2006; 84: 1253]. Am J Clin Nutr 2006; 84: 18–28.
Wang H, Xia N, Yang Y, Peng DQ . Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysys of randomized controlled trials. Lipids Health Dis 2012; 11: 11–42.
American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care 2012; 35: S11–S63.
Davidson MB, Duran P, Lee ML, Friedman TC . High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 2013; 36: 260–266.
Acknowledgements
We acknowledge the support of the staff of the diabetes clinic for their support in data collection, Vijay Kumar and Ponnu Andrew Prem Kumar from the laboratory in specimen processing and Joseph George for IT support at Rashid Center for Diabetes and Research.
Clinical trial registry: ClinicalTrials.gov Identifier: NCT02101151
Funding: This clinical trial was supported by grants from Rashid Center for Diabetes and Research and Global Health Partner. Solgar, NJ, USA, supplied the vitamin D3 capsules and Compound Pharmacy, and Dubai, UAE, supplied placebo capsules used in the present study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Authors’ contribution
AS, SMA, MC, YT and SA designed the research, AS, SMA, YT, SHA, MG, HHS and SA conducted the research, AS and SA analyzed the data, AS wrote the paper and AS and SA had primary responsibility for the final content. All authors read and approved the final manuscript.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Sadiya, A., Ahmed, S., Carlsson, M. et al. Vitamin D supplementation in obese type 2 diabetes subjects in Ajman, UAE: a randomized controlled double-blinded clinical trial. Eur J Clin Nutr 69, 707–711 (2015). https://doi.org/10.1038/ejcn.2014.251
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.251
This article is cited by
-
What is the impact of vitamin D supplementation on glycemic control in people with type-2 diabetes: a systematic review and meta-analysis of randomized controlled trails
BMC Endocrine Disorders (2023)
-
Serum 25-hydroxyvitamin D level is associated with short-term glycemic variability metrics derived from continuous glucose monitoring in T2DM
Scientific Reports (2023)
-
Could nutrient supplements provide additional glycemic control in diabetes management? A systematic review and meta-analysis of randomized controlled trials of as an add-on nutritional supplementation therapy
Archives of Pharmacal Research (2022)
-
Vitamin D3-fortified milk did not affect glycemic control, lipid profile, and anthropometric measures in patients with type 2 diabetes, a triple-blind randomized clinical trial
European Journal of Clinical Nutrition (2018)
-
Impact of vitamin D replacement in adults and elderly in the Middle East and North Africa: a systematic review and meta-analysis of randomized controlled trials
Osteoporosis International (2017)