Abstract
Background/Objectives:
In India, 75% of children <5 years of age have anemia. The National Nutritional Anemia Control Program (NNACP) recommends 20 mg iron and 100 μg folic acid (IFA) supplementation for 100 days/year, but still anemia prevalence has remained high. To accelerate the progress, suggestions include increase in IFA to therapeutic dose or supervised weekly supplementation to improve compliance. The objectives of this study was to compare the hemoglobin response with two dosages of daily (20 mg iron and 100 μg folic acid, or 40 mg iron and 200 μg folic acid) and weekly (40 mg iron and 200 μg folic acid) IFA supplementation in children of 3–5 years of age with mild or moderate anemia (hemoglobin 7–10 g/dl).
Subjects/Methods:
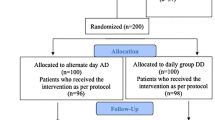
Community-based cluster randomized control trial in nine adjoining Anganwadi Centers. Four hundred twenty six enrolled participants received directly supervised IFA tablet supplementation as per the above three groups. After 100 days, the number of available subjects in the NNACP daily dose (A), daily dose doubled (B) and weekly dose (C) groups were 112, 114 and 110, respectively. Hemoglobin was estimated at baseline, 50 and 100 days by the Cynmeth hemoglobin method.
Results:
At 50 days, there were no differences between the three groups, but at 100 days, adjusted hemoglobin was lowered with weekly supplementation. The mean (95% confidence interval) hemoglobin (g/dl) differences were: (i) A–B: −0.05 (−0.17, 0.05), (ii) A–C: −0.38 (−0.50, −0.27) and (iii) B–C: −0.33, (−0.45, −0.21). Anemia reduction was 18.8%, 18.4% and 10.9%, respectively, in the three groups.
Conclusion:
Directly supervised IFA supplementation at the NNACP or double dose is equally efficacious but superior to weekly regimen.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
World Health Organization and United Nations Children’s Fund. Focusing on anaemia: towards an integrated approach for effective anaemia control. World Health Organization: Geneva, 2004: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/en/index.html (accessed 22 October 2012).
Allen L, Sabel JC . Prevalence and causes of nutritional anemia’s. In: Ramakrishnan U, (ed) Nutritional Anemia’s. CRC Press: London, 2001.
The World Health Report 1998. Life in the 21st Century. A Vision for All. World Health Organization, Geneva, 1998.
Preventing iron deficiency in women and children. Technical consensus on key issues. UNICEF, UNU, WHO, MI, Technical Group International Nutrition Foundation, USA, 7–9 October 1998.
International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005-06, Vol. 1. IIPS: Mumbai, India, 2007.
Kapil U . National nutrition supplementation programmes. Indian Pediatr 1992; 29: 1601–1613.
Kumar N, Shekhar C, Kumar P, Kundu AS . Kuppuswamy’s socioeconomic status scale—Updating for 2007. Indian J Pediatr 2007; 74: 1131–1132.
Lerner NB, Sills R . Iron deficiency anemia. In: Nelson Textbook of Pediatrics 19th edn. Kliegman RM, Stanton BF St., Geme JW, Schor NF, Behrman RE, (eds), Saunders: Philadelphia, 2011, pp 1655–1658).
De-Regil LM, Jefferds MED, Sylvetsky AC, Dowswell T . Intermittent iron supplementation for improving nutrition and development in children under 12 years of age. Cochrane Database Syst Rev 2011 ((12))CD009085 doi:10.1002/14651858.CD009085.pub2.
Hirve S, Bhave S, Bavdekar A, Naik S, Pandit A, Schauer C et al. Low dose ‘Sprinkles’—an innovative approach to treat iron deficiency anemia in infants and young children. Indian Pediatr 2007; 44: 91–100.
Christofides A, Asante KP, Schauer C, Sharieff W, Owusu-Agyei S, Zlotkin S . Multi-micronutrient Sprinkles including a low dose of iron provided as microencapsulated ferrous fumarate improves haematologic indices in anaemic children: a randomized clinical trial. Matern Child Nutr 2006; 2: 169–180.
Roberfroid D, Huybregts L, Habicht JP, Lanou H, Henry MC, Meda N et al. Randomized controlled trial of 2 prenatal iron supplements: is there a dose-response relation with maternal hemoglobin? Am J Clin Nutr 2011; 93: 1012–1018.
Casgrain A, Collings R, Harvey LJ, Hooper L, Fairweather-Tait SJ . Effect of iron intake on iron status: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2012; 96: 768–780.
Gera T, Sachdev HPS, Nestel P, Sachdev SS . Effect of iron supplementation on hemoglobin response in children: Systematic review of randomised controlled trials. J Pediatr Gastroenterol Nutr 2007; 44: 468–486.
Prevalence of Micronutrient Deficiencies. National Nutrition Monitoring Bureau Technical Report No. 22. National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India, 2003. http://nnmbindia.org/NNMB%20MND%20REPORT%202004-Web.pdf (accessed 22 October 2012).
Acknowledgements
We are extremely grateful to Indian Council of Medical Research, New Delhi for providing us the financial grant for conducting the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kapil, U., Sachdev, H., Dwivedi, S. et al. Relative efficacy of weekly and two differing doses of daily iron–folate supplementation in improving hemoglobin in mild and moderately anemic children between 3 and 5 years of age: a cluster randomized trial. Eur J Clin Nutr 67, 343–347 (2013). https://doi.org/10.1038/ejcn.2013.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.13