Abstract
Background/Objectives:
It is well established that combining a reduced calorie, low-fat diet with the lipase inhibitor orlistat results in significantly greater weight loss than placebo plus diet. This weight loss is accompanied by changes in adipose tissue (AT) distribution. As 60 mg orlistat is now available as an over-the-counter medication, the primary objective of this study was to determine whether 60 mg orlistat is effective as a weight loss option in a free-living community population with minimal professional input.
Methods:
AT and ectopic lipid content were measured using magnetic resonance imaging and 1H MR spectroscopy, respectively, in 27 subjects following 3 months treatment with orlistat 60 mg and a reduced calorie, low-fat diet.
Results:
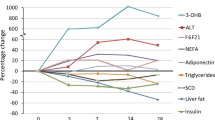
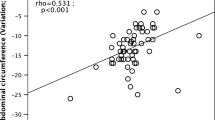
Significant reductions in intra-abdominal AT (−10.6%, P=0.023), subcutaneous (−11.7% P<0.0001) and pericardial fat (−9.8%, P=0.034) volumes and intrahepatocellular lipids (−43.3%, P=0.0003) were observed. These changes in body fat content and distribution were accompanied by improvements in plasma lipids and decreases in blood pressure and heart rate.
Conclusion:
These findings suggest that over-the-counter 60 mg orlistat, in combination with the type of advice a subject could expect to be given when obtaining 60 mg orlistat in a community setting, does indeed result in potentially clinically beneficial changes in body composition and risk factors for metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Anderson J, Schwartz S, Hauptman J, Boldrin M, Bansal V, Hale C (2006). Low-dose orlistat effects on body weight of mildly to moderately overweight individuals: a 16 week, double-blind, placebo controlled trial. Ann Pharmacother 40, 1717–1723.
Anderson JW (2007). Orlistat for the management of overweight individuals and obesity: a review of potential for the 60 mg, over-the-counter dosage. Expert Opin Pharmacother 8, 1733–1742.
Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S et al. (1997). Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-h urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol 26, S137–S151.
Bosy-Westphal A, Booke CA, Blöcker T, Kossel E, Goele K, Later W et al. (2010). Measurement site for waist circumference affects its accuracy as an index of visceral and abdominal subcutaneous fat in a Caucasian population. J Nutr 140, 954–961.
Brass EP (2001). Changing the status of drugs from prescription to over-the-counter availability. N Engl J Med 13, 810–816.
Brochu M, Tchernof A, Turner AN, Ades PA, Poehlman ET (2003). Is there a threshold of visceral fat loss that improves the metabolic profile in obese postmenopausal women? Metabolism 52, 599–604.
Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J (2005). Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA 293, 2873–2883.
Chaston TB, Dixon JB (2008). Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 32, 619–628.
Department of Health (1991). Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. HMSO: London.
Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E et al. (2008). Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28, 1039–1049.
Dixon JB, Strauss BJ, Laurie C, O’Brien PE (2007). Changes in body composition with weight loss: obese subjects randomized to surgical and medical programs. Obesity 15, 1187–1198.
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW et al. (2009). Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 106, 15430–15435.
Goldstein DJ (1992). Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 16, 397–415.
Hamdy O, Porramatikul S, Al-Ozairi E (2006). Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2, 367–373.
Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR (2000). Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 9, 160–167.
Hussein O, Grosovski M, Schlesinger S, Szvalb S, Assy N (2007). Orlistat reverses fatty infiltration and improves hepatic fibrosis in obese patients with nonalcoholic steatohepatitis (NASH). Dig Dis Sci 52, 2512–2519.
Iacobellis G, Singh N, Wharton S, Sharma AM (2008). Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 16, 1693–1697.
Kelley DE, Kuller LH, McKolanis TM, Harper P, Mancino J, Kalhan S (2004). Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care 27, 33–40.
Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH (1999). The disease burden associated with overweight and obesity. JAMA 282, 1523–1529.
Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R et al. (2001). Java-based graphical user interface for the MRUI quantitation package. MAGMA 12, 141–152.
National Institutes of Health (1998). Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 6, 51S–209S.
Oka R, Miura K, Sakurai M, Nakamura K, Yagi K, Miyamoto S et al. (2009). Comparison of waist circumference with body mass index for predicting abdominal adipose tissue. Diabetes Res Clin Pract 83, 100–105.
Rico-Sanz J, Thomas EL, Jenkinson G, Mierisová S, Iles R, Bell JD (1999). Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by (1)H-MRS. J Appl Physiol 87, 2068–2072.
Rofsky NM, Lee VS, Laub G, Pollack MA, Krinsky GA, Thomasson D et al. (1999). Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology 212, 876–884.
Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL et al. (2004). Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 12, 789–798.
Rössner S, Sjöström L, Noack R, Meinders AE, Noseda G (2000). Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res 8, 49–61.
Schwartz SM, Bansal VP, Hale C, Rossi M, Engle JP (2008). Compliance, behavior change, and weight loss with orlistat in an over-the-counter setting. Obesity 16, 623–629.
Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD et al. (2006). Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol 12, 5813–5819.
Thomas EL, Brynes AE, McCarthy J, Goldstone AP, Hajnal JV, Saeed N et al. (2000). Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids 35, 769–776.
Thomas EL, Hamilton G, Patel N, O’Dwyer R, Doré CJ, Goldin RD et al. (2005). Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut 54, 122–127.
Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G et al. (1998). Magnetic resonance imaging of total body fat. J Appl Physiol 85, 1778–1785.
Tiikkainen M, Bergholm R, Rissanen A, Aro A, Salminen I, Tamminen M et al. (2004). Effects of equal weight loss with orlistat and placebo on body fat and serum fatty acid composition and insulin resistance in obese women. Am J Clin Nutr 79, 22–30.
World Health Organisation (2006). Obesity and overweight. Fact Sheet No. 311, September. Available at http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
Acknowledgements
This study was funded by GlaxoSmithKline, manufacturer of alli. ELT and JDB also acknowledge funding from the UK MRC and NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AM, RN, AWR, GG, BD, PMM, ESB, RGM, SMS and JD Beaver are employees of GSK. JD Bell and GF have received consultancy fees from GSK. ELT declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Thomas, E., Makwana, A., Newbould, R. et al. Pragmatic study of orlistat 60 mg on abdominal obesity. Eur J Clin Nutr 65, 1256–1262 (2011). https://doi.org/10.1038/ejcn.2011.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2011.108