Abstract
Objectives:
Drinking green tea is associated with many health benefits, including increased fat oxidation. We tested the hypothesis that epigallocatechin-3-gallate (EGCG), the main green tea catechin, increases fat oxidation in obese men.
Methods:
Ten healthy overweight/obese males (body mass index 31.3±0.8 kg/m2) were studied in a randomized, placebo-controlled, double-blind crossover trial. Study supplements were low EGCG (300 mg), high EGCG (600 mg), caffeine (200 mg), EGCG/caffeine (300 mg/200 mg) or placebo and were taken orally for 3 days. At the third day of supplementation, O2 consumption and CO2 production was measured by indirect calorimetry to assess energy expenditure and fat oxidation over 4 h each after overnight fasting and after a standardized test meal.
Results:
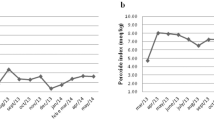
Energy expenditure was not affected by any supplementation, neither after overnight fasting nor after the test meal. During the first 2 h after overnight fasting, fat oxidation increased by 7.7 (not significant, NS), 15.2 (NS), 26.3 (P<0.05 vs placebo) and 35.4% (P<0.01 vs placebo and low EGCG), for low EGCG, high EGCG, caffeine and EGCG/caffeine, respectively. During the first 2 h after the meal, the mean increase in fat oxidation was 33.3 (P<0.05 vs placebo), 20.2 (NS), 34.5 (P<0.05 vs placebo) and 49.4% (P<0.05 vs placebo) for low EGCG, high EGCG, caffeine and EGCG/caffeine, respectively.
Conclusions:
Low EGCG increases postprandial fat oxidation in obese men and this to the same extent as 200 mg caffeine, whereas high EGCG does not exert this effect. Fasting fat oxidation is increased only by caffeine (with or without EGCG). There is no synergism of low EGCG and 200 mg caffeine. Energy expenditure is not affected by EGCG.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Acheson KJ, Zahorska-Markiewicz B, Pittet P, Anantharaman K, Jequier E (1980). Caffeine and coffee: their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am J Clin Nutr 33, 989–997.
Arch JR, Wilson S (1996). Prospects for beta 3-adrenoceptor agonists in the treatment of obesity and diabetes. Int J Obes Relat Metab Disord 20, 191–199.
Arciero PJ, Bougopoulos CL, Nindl BC, Benowitz NL (2000). Influence of age on the thermic response to caffeine in women. Metabolism 49, 101–107.
Astrup A, Toubro S, Cannon S, Hein P, Breum L, Madsen J (1990). Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr 51, 759–767.
Belza A, Toubro S, Astrup A (2009). The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur J Clin Nutr 63, 57–64.
Berube-Parent S, Pelletier C, Dore J, Tremblay A (2005). Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br J Nutr 94, 432–436.
Blaak EE (2000). Adrenergically stimulated fat utilization and ageing. Ann Med 32, 380–382.
Borchardt RT, Huber JA (1975). Catechol O-methyltransferase. 5. Structure-activity relationships for inhibition by flavonoids. J Med Chem 18, 120–122.
Boschmann M, Steiniger J, Franke G, Birkenfeld AL, Luft FC, Jordan J (2007). Water drinking induces thermogenesis through osmosensitive mechanisms. J Clin Endocrinol Metab 92, 3334–3337.
Boschmann M, Steiniger J, Hille U, Tank J, Adams F, Sharma AM et al. (2003). Water-induced thermogenesis. J Clin Endocrinol Metab 88, 6015–6019.
Bracco D, Ferrarra JM, Arnaud MJ, Jequier E, Schutz Y (1995). Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am J Physiol 269, 671–678.
Brown CM, Dulloo AG, Montani JP (2006). Water-induced thermogenesis reconsidered: the effects of osmolality and water temperature on energy expenditure after drinking. J Clin Endocrinol Metab 91, 3598–3602.
Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC (2006). Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome—a randomized placebo-controlled trial. J Soc Gynecol Investig 13, 63–68.
Chantre P, Lairon D (2002). Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine 9, 3–8.
Dulloo AG (1993a). Ephedrine, xanthines and prostaglandin-inhibitors: actions and interactions in the stimulation of thermogenesis. Int J Obes Relat Metab Disord 17 (Suppl 1), 35–40.
Dulloo AG (1993b). Strategies to counteract readjustments toward lower metabolic rates during obesity management. Nutrition 9, 366–372.
Dulloo AG (1998). Spicing fat for combustion. Br J Nutr 80, 493–494.
Dulloo AG, Geissler CA, Horton T, Collins A, Miller DS (1989). Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am J Clin Nutr 49, 44–50.
Dulloo AG, Seydoux J, Girardier L (1992). Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: adenosine antagonism or phosphodiesterase inhibition? Metabolism 41, 1233–1241.
Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M et al. (1999). Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 70, 1040–1045.
Ferrannini E (1988). The theoretical bases of indirect calorimetry: a review. Metabolism 37, 287–301.
Flegal KM, Carroll MD, Ogden CL, Johnson CL (2002). Prevalence and trends in obesity among US adults, 1999–2000. Jama 288, 1723–1727.
Hase T, Komine Y, Meguro S, Takeda Y, Takahashi H, Matsui Y et al. (2001). Anti-obesity effects of tea catechins in humans. J Oleo Sci 50, 599–605.
Henry CJ, Emery B (1986). Effect of spiced food on metabolic rate. Hum Nutr Clin Nutr 40, 165–168.
Horton TJ, Geissler CA (1996). Post-prandial thermogenesis with ephedrine, caffeine and aspirin in lean, pre-disposed obese and obese women. Int J Obes Relat Metab Disord 20, 91–97.
Hung PF, Wu BT, Chen HC, Chen YH, Chen CL, Wu MH et al. (2005). Antimitogenic effect of green tea (-)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the ERK and Cdk2 pathways. Am J Physiol Cell Physiol 288, 1094–1108.
Jordan J, Shannon JR, Diedrich A, Black B, Robertson D, Biaggioni I (2004). Water potentiates the pressor effect of ephedra alkaloids. Circulation 109, 1823–1825.
Juhel C, Armand M, Pafumi Y, Rosier C, Vandermander J, Lairon D (2000). Green tea extract (AR25(R)) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J Nutr Biochem 11, 45–51.
Kajimoto O, Kajimoto Y, Yabune M, Nakamura T, Kotani K (2006). Tea catechins with a galloyl moiety reduce body weight and fat. J Health Sci 1, 161–171.
Klaus S, Pultz S, Thone-Reineke C, Wolfram S (2005). Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes Relat Metab Disord 29, 615–623.
Landsberg L, Young JB (1993). Sympathoadrenal activity and obesity: physiological rationale for the use of adrenergic thermogenic drugs. Int J Obes Relat Metab Disord 17 (Suppl 1), 29–34.
Liles JT, Baber SR, Deng W, Porter JR, Corll C, Murthy SN et al. (2007). Pressor responses to ephedrine are not impaired in dopamine beta-hydroxylase knockout mice. Br J Pharmacol 150, 29–36.
Nagao T, Hase T, Tokimitsu I (2007). A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 15, 1473–1483.
Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y et al. (2005). Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr 81, 122–129.
Ota N, Soga S, Shimotoyodome A, Inaba M, Murase T, Tokimitsu I (2005). Effects of combination of regular exercise and tea catechins intake on energy expenditure in humans. J Health Sci 51, 233–236.
Raederstorff DG, Schlachter MF, Elste V, Weber P (2003). Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem 14, 326–332.
Rhodes M (1996). Physiologically-active compounds in plant foods: an overview. Proc Nutr Soc 55, 371–384.
Rudelle S, Ferruzzi MG, Cristiani I, Moulin J, Mace K, Acheson KJ et al. (2007). Effect of a thermogenic beverage on 24-h energy metabolism in humans. Obesity (Silver Spring) 15, 349–355.
Rumpler W, Seale J, Clevidence B, Judd J, Wiley E, Yamamoto S et al. (2001). Oolong tea increases metabolic rate and fat oxidation in men. J Nutr 131, 2848–2852.
Scholz E, Bertram B, Kuntze O (1995). Camellia sinensis—der teestrauch, inhaltsstoffe und wirkungen von grünem und schwarzem tee, portrait einer arzneipflanze. Zeitschrift für Phytotherapie 4, 231–246.
Tsuchida T, Itakura H, Nakamura H (2002). Reduction of body fat in humans by long-term ingestion of catechins. Prog Med 9, 2189–2203.
Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard CAS et al. (2003). A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res 31, 88–101.
Venables MC, Hulston CJ, Cox HR, Jeukendrup AE (2008). Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr 87, 778–784.
Westerterp-Plantenga MS, Lejeune MP, Kovacs EM (2005). Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res 13, 1195–1204.
Wolfram S, Raederstorff D, Wang Y, Teixeira SR, Elste V, Weber P (2005). TEAVIGO (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann Nutr Metab 49, 54–63.
Yang CS, Landau JM (2000). Effects of tea consumption on nutrition and health. J Nutr 130, 2409–2412.
Yang M, Wang C, Chen H (2001). Green, oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem 12, 14–20.
Yoshioka M, St-Pierre S, Suzuki M, Tremblay A (1998). Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br J Nutr 80, 503–510.
Acknowledgements
The authors thank Richard Gössl (DSM Nutritional Products, Kaiseraugst, Switzerland) for expert technical assistance. Gritt Stoffels and Stefan Engeli (University Hospital Charité Campus Buch, Berlin, Germany) were involved in the recruitment and assessment of volunteers in all human studies. The authors thank Dr Frida Dangardt (Sahlgrenska Academy at the University of Gothenburg, Sweden) for assisting in statistical analysis and Dr Marcella Trembley (DSM Nutritional Products, Kaiseraugst, Switzerland) for critical reading of the paper. Funding for this study was provided by DSM Nutritional Products.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
FT is employed by DSM Nutritional Products. The results of this study have no effect on his employment status.
Additional information
Contributors: FT, MB, AB and JJ designed the research; GF, JB, AB, FA and MB performed the research; FT and MB analyzed the data; and FT and MB wrote the paper.
Rights and permissions
About this article
Cite this article
Thielecke, F., Rahn, G., Böhnke, J. et al. Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: a pilot study. Eur J Clin Nutr 64, 704–713 (2010). https://doi.org/10.1038/ejcn.2010.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2010.47
Keywords
This article is cited by
-
The polyphenol epigallocatechin gallate lowers circulating catecholamine concentrations and alters lipid metabolism during graded exercise in man: a randomized cross-over study
European Journal of Nutrition (2023)
-
Effect of tea catechins with caffeine on energy expenditure in middle-aged men and women: a randomized, double-blind, placebo-controlled, crossover trial
European Journal of Nutrition (2020)
-
Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials
European Journal of Clinical Nutrition (2016)
-
A 3-day EGCG-supplementation reduces interstitial lactate concentration in skeletal muscle of overweight subjects
Scientific Reports (2015)
-
Short-term supplementation with a specific combination of dietary polyphenols increases energy expenditure and alters substrate metabolism in overweight subjects
International Journal of Obesity (2014)