Abstract
Objective:
To evaluate the influence of oxalic acid (OA) on nonhaem iron absorption in humans.
Design:
Two randomized crossover stable iron isotope absorption studies.
Setting:
Zurich, Switzerland.
Subjects:
Sixteen apparently healthy women (18–45 years, <60 kg body weight), recruited by poster advertizing from the staff and student populations of the ETH, University and University Hospital of Zurich, Switzerland. Thirteen subjects completed both studies.
Methods:
Iron absorption was measured based on erythrocyte incorporation of 57Fe or 58Fe 14 days after the administration of labelled meals. In study I, test meals consisted of two wheat bread rolls (100 g) and either 150 g spinach with a native OA content of 1.27 g (reference meal) or 150 g kale with a native OA content of 0.01 g. In study II, 150 g kale given with a potassium oxalate drink to obtain a total OA content of 1.27 g was compared to the spinach meal.
Results:
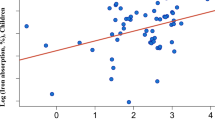
After normalization for the spinach reference meal absorption, geometric mean iron absorption from wheat bread rolls with kale (10.7%) did not differ significantly from wheat rolls with kale plus 1.26 g OA added as potassium oxalate (11.5%, P=0.86). Spinach was significantly higher in calcium and polyphenols than kale and absorption from the spinach meal was 24% lower compared to the kale meal without added OA, but the difference did not reach statistical significance (P>0.16).
Conclusion:
Potassium oxalate did not influence iron absorption in humans from a kale meal and our findings strongly suggest that OA in fruits and vegetables is of minor relevance in iron nutrition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ballot D, Baynes RD, Bothwell TH, Gillooly M, MacFarlane BJ, MacPhail AP et al. (1987). The effects of fruit juices and fruits on the absorption of iron from a rice meal. Br J Nutr 57, 331–343.
Bjorn-Rasmussen E, Hallberg L, Walker RB (1973). Food iron absorption in man. II. Isotopic exchange of iron between labeled foods and between a food and an iron salt. Am J Clin Nutr 26, 1311–1319.
Bohn T (2003). Magnesium Absorption in Humans. Department of Agricultural and Food Sciences. Zurich, ETH Zurich, 200.
Brown E, Hopper Jr J, Hodges Jr JL, Bradley B, Wennesland R, Yamauchi H (1962). Red cell, plasma, and blood volume in the healthy women measured by radiochromium cell-labeling and hematocrit. J Clin Invest 41, 2182–2190.
Brune M, Rossander L, Hallberg L (1989). Iron absorption and phenolic compounds: importance of different phenolic structures. Eur J Clin Nutr 43, 547–558.
Chai W, Liebman M (2005). Effect of different cooking methods on vegetable oxalate content. J Agric Food Chem 53, 3027–3030.
Chun OK, Smith N, Sakagawa A, Lee CY (2004). Antioxidant properties of raw and processed cabbages. Int J Food Sci Nutr 55, 191–199.
Cook JD, Layrisse M, Martinez-Torres C, Walker R, Monsen E, Finch CA (1972). Food iron absorption measured by an extrinsic tag. J Clin Invest 51, 805–815.
Cook JD, Monsen ER (1977). Vitamin C, the common cold, and iron absorption. Am J Clin Nutr 30, 235–241.
Fairweather-Tait SJ, Dainty J (2002). Use of stable isotopes to assess the bioavailability of trace elements: a review. Food Addit Contam 19, 939–947.
Gillooly M, Bothwell TH, Torrance JD, MacPhail AP, Derman DP, Bezwoda WR et al. (1983). The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br J Nutr 49, 331–342.
Gordon DT, Chao LS (1984). Relationship of components in wheat bran and spinach to iron bioavailability in the anemic rat. J Nutr 114, 526–535.
Hallberg L, Brune M, Erlandsson M, Sandberg AS, Rossander-Hulten L (1991). Calcium: effect of different amounts on nonheme- and heme-iron absorption in humans. Am J Clin Nutr 53, 112–119.
Hallberg L, Brune M, Rossander L (1986). Effect of ascorbic acid on iron absorption from different types of meals. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum Nutr Appl Nutr 40, 97–113.
Heaney RP, Weaver CM (1989). Oxalate: effect on calcium absorbability. Am J Clin Nutr 50, 830–832.
Heaney RP, Weaver CM, Recker RR (1988). Calcium absorbability from spinach. Am J Clin Nutr 47, 707–709.
Hodgkinson A, Zarembski PM (1968). Oxalic acid metabolism in man: a review. Calcif Tissue Res 2, 115–132.
Holmes RP, Kennedy M (2000). Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int 57, 1662–1667.
Honow R, Hesse A (2002). Comparison of extraction methods for the determination of soluble and total oxalate in foods by HPLC-enzyme-reactor. Food Chem 78, 511–521.
Hosain F, Marsaglia G, Finch CA (1967). Blood ferrokinetics in normal man. J Clin Invest 46, 1–9.
Hurrell RF, Reddy M, Cook JD (1999). Inhibition of nonhaem iron absorption in man by polyphenolic-containing beverages. Br J Nutr 81, 289–295.
Kasidas GP, Rose GA (1980). Oxalate content of some common foods: determination by an enzymatic method. J Hum Nutr 34, 255–266.
Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF (1994). A double stable-isotope technique for measuring iron-absorption in infants. Br J Nutr 71, 411–424.
Kelsay JL, Prather ES (1983). Mineral balances of human subjects consuming spinach in a low-fiber diet and in a diet containing fruits and vegetables. Am J Clin Nutr 38, 12–19.
Makower RU (1970). Extraction and determination of phytic acid in beans (phaeolus vulgaris). Cereal Chem 47, 288–295.
Ninfali P, Bacchiocca M (2003). Polyphenols and antioxidant capacity of vegetables under fresh and frozen conditions. J Agric Food Chem 51, 2222–2226.
Nyyssönen K, Salonen JT, Parviainen MT (1992). Ascorbic acid. In: A De Lenheer, WE Lambert and HJ Nelis (eds). Modern Chromatographic Analysis of Vitamins. Marcel Dekker: New York. pp 235–260.
Salovaara S, Sandberg AS, Andlid T (2002). Organic acids influence iron uptake in the human epithelial cell line Caco-2. J Agric Food Chem 50, 6233–6238.
Sapers GM, Douglas Jr FW, Ziolkowski MA, Miller RL, Hicks KB (1990). Determination of ascorbic acid, dehydro-ascorbic acid and ascorbic acid-2-phosphate in infiltrated apple and potato tissue by HPLC. J Chromatogr 503, 431–436.
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol 299, 152–178.
Souci SW, Fachmann W, Kraut H (1994). Food Composition and Nutrition Tables. Medpharm Scientific Publishers: Stuttgart.
Van Campen DR, Welch RM (1980). Availability to rats of iron from spinach: effects of oxalic acid. J Nutr 110, 1618–1621.
von Unruh GE, Voss S, Sauerbruch T, Hesse A (2004). Dependence of oxalate absorption on the daily calcium intake. J Am Soc Nephrol 15, 1567–1573.
Walczyk T (1997). Iron isotope ratio measurements by negative thermal ionisation mass spectrometry using FeF-4 molecular ions. Int J Mass Spectrom Ion Processes 161, 217–227.
Walczyk T, Davidsson L, Zavaleta N, Hurrell RF (1997). Stable isotope labels as a tool to determine the iron absorption by Peruvian school children from a breakfast meal. Fresenius J Anal Chem 359, 445–449.
Zarembski PM, Hodgkinson A (1962). The oxalic acid content of English diets. Br J Nutr 16, 627–634.
Acknowledgements
The help of Eberhard Denk, Ralf Biebinger and Ines Egli in performing the human studies is greatly appreciated. Special thanks go to Christophe Zeder for preparing the isotope solutions and helping with data analysis, to Karin Hotz for meal iron and calcium determination, to Charlotte Züllig and Marlies Krähenbühl for taking blood samples, and to the volunteers for participating in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributors: SSgB was involved in the planning of the study, carried out the study, measured iron status parameters, analysed the data and wrote the first draft of the manuscript. TW contributed to the study design, supervised the meal administration and revised the manuscript draft. SR performed the mass spectrometric iron isotope measurements. RFH supervised the project, contributed to the study design and critically revised the manuscript draft.
Rights and permissions
About this article
Cite this article
genannt Bonsmann, S., Walczyk, T., Renggli, S. et al. Oxalic acid does not influence nonhaem iron absorption in humans: a comparison of kale and spinach meals. Eur J Clin Nutr 62, 336–341 (2008). https://doi.org/10.1038/sj.ejcn.1602721
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1602721