Abstract
Cereal grains and their products provide around 30% of total energy intake in British adults, (much more than any of the other major food groups). Coronary heart disease (CHD) is the largest single cause of death in Britain and many other Western countries. This review examines the question whether there is a relation between cereal consumption and CHD.
Several of the nutrients in cereals have known potential for reducing risk factors for CHD: the linoleic acid, fibre, vitamin E, selenium and folate. Cereals also contain phytoestrogens of the lignan family and several phenolic acids with antioxidant properties. Processing generally reduces the content of these nutrients and bioprotective substances. Although cereals at the farm gate are very low in salt, processed cereal foods, eg bread and some breakfast cereals, are high-salt foods and thus could contribute to raising blood pressure.
Human experiments have clearly shown that oat fibre tends to lower plasma total and LDL cholesterol but wheat fibre does not. Rice bran and barley may also lower cholesterol but most people do not eat enough barley to have an effect. Cereal foods with low glycaemic index such as pasta and oats are beneficial for people with diabetes and might lower plasma lipids.
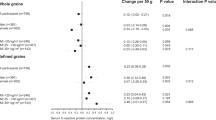
Between 1996 and 2001 an accumulation of five very large cohort studies in the USA, Finland and Norway have all reported that subjects consuming relatively large amounts of whole grain cereals have significantly lower rates of CHD. This confirms an earlier report from a small British cohort. The protective effect does not seem to be due to cholesterol-lowering. While cohort studies have shown this consistent protective effect of whole grain cereals, there has been (only one) randomised controlled secondary prevention trial of advice to eat more cereal fibre. In this there was no reduction of the rate of reinfarction. The trial had some weaknesses, eg there were eight different diets, compliance was not checked objectively, and duration was for only 2 y.
It appears valid to make health claims (as now permitted by the US FDA) that whole grain cereal foods and oat meal or bran may reduce the risk of CHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Notes
All the references for this section are in Truswell and Beynen (1992).
References
Anderson JW, Story L, Sieling B, Chen WJL, Petro MS & Story J . 1984a Hypocholesterolemic effects of oat bran or bean intake for hypercholesterolemic men Am. J. Clin. Nutr. 40: 1146–1155
Anderson JW, Story L, Sieling B & Chen WJL . 1984b Hypocholesterolemic effects of high-fibre diets rich in water-soluble plant fibres J. Can. Diet. Assoc. 45: 14–148
Anderson JW, Spencer DB, Hamilton CC, Smith SF, Tietyen J, Bryant CA & Oeltgen P . 1990 Oat-bran cereal lowers serum total and LDL cholesterol in hypercholesterolemic men Am. J. Clin. Nutr. 52: 495–499
Anderson JW, Gilinsky NH, Deakins DA, Smith SF, O'Neal DS, Dillon DW & Oeltgen P . 1991 Lipid responses of hypercholesterolemic men to oat-bran and wheat-bran intake Am. J. Clin. Nutr. 54: 678–683
Birkeland KI, Gullestad L, Falch D & Torsvik H . 1990 Oat bran and serum cholesterol New Engl. J. Med. 322: 1748–1749
Braaten JT, Wood PJ, Scott FW, Wolynetz MS, Lowe MK, Bradley-White P & Collins MW . 1994 Oat β-glucan reduces blood cholesterol in hypercholesterolemic subjects Eur. J. Clin. Nutr. 48: 465–474
Brand Miller J . 1994 Importance of glycemic index in diabetes Am. J. Clin. Nutr. 59: 747S–752S
Bremer JM, Scott RS & Lintott CJ . 1990 Oat bread versus bread for lipid lowering Proc. Nutrition Soc. N.Z. 15: 171–172
Brown L, Rosner B, Willett WW & Sacks FM . 1999 Cholesterol-lowering effects of dietary fibre: a meta-analysis Am. J. Clin. Nutr. 69: 30–42
Burr ML & Sweetnam PM . 1982 Vegetarianism, dietary fiber and mortality Am. J. Clin. Nutr. 36: 873–877
Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC & Deadman NM . 1989 Effects of changes in fat, fish and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet ii: 757–761
Cleave TL . 1974 The Saccharine Disease. Conditions Caused by the Taking of Refined Carbohydrates, Such as Sugar and White Flour John Wright: Bristol
Davidson MH, Dugan LD, Burns JH, Bova J, Story K & Drennan KB . 1991 The hypocholesterolemic effects of β-glucan in oatmeal and oat bran J.A.M.A. 265: 1833–1839
De Groot AP, Luyken R & Pikaar NA . 1963 Cholesterol-lowering effect of rolled oats Lancet ii: 303–304
Demark-Wahnefried W, Bowering J & Cohen PS . 1990 Reduced serum cholesterol with dietary change using fat-modified and oat bran supplemented diet J. Am. Diet. Ass. 90: 223–229
Denton D, Weisinger R, Mundy NI, Wickings EJ, Dixson A, Moisson P et al. 1995 The effect of increased salt intake on blood pressure of chimpanzees Nature Med. 1: 1009–1016
Elwood PC, Burr ML & Sweetnam PM . 1992 Fish, fibre and heart disease In Coronary Heart Disease Epidemiology. From Aetiology to Public Health, M Marmot & P Eliott (eds). 203–216 Oxford Medical Publications: Oxford
Fehily AM, Yarnell JWG, Sweetnam PM & Elwood PC . 1993 Diet and incident ischaemic heart disease: The Caerphilly study Br. J. Nutr. 69: 303–314
Flanagan M, Little C, Milliken J et al. 1980 The effects of diet on high density lipoprotein J. Human Nutr. 34: 34–45
Food and Drug Administration . 1999 Washington DC Health Claims Chart-Authorised Claims
Foster-Powell K & Brand Miller J . 1995 International tables of glycemic index Am. J. Clin. Nutr. 62: 871S–893S
Gariot P, Digby JP, Genton P, Lambert D, Bau RMH & Debry G . 1986 Long-term effect of brain ingestion on lipid metabolism in healthy men Ann. Nutr. Metab. 30: 369–373
Gold KV & Davidson DM . 1988 Oat bran as a cholesterol-reducing dietary adjunct in a young, healthy population West. J. Med. 148: 299–302
Gregory J, Foster K, Tyler H & Wiseman M . 1990 The Dietary and Nutritional Survey of British Adults HMSO: London
Hamilton CC, Tietyen J, Spencer BA & Anderson JW . 1989 Serum lipid responses of hypercholesterolemic men to a ready to eat oat bran cereal J. Am. Diet. Ass. 89 Suppl: A19
Hankey GJ & Eikelboom JW . 1999 Review. Homocysteine and vascular diseases Lancet 354: 407–413
Hansen RG . 1973 An index of food quality Nutrition Reviews 31: 1–7
Hansen RG, Wyse BW & Sorenson AW . 1979 Nutritional Quality Index of Foods Avi Publishing, Connecticut: Westport
Hegsted M, Windhauser MM, Lester SB & Morris K . 1990 Stabilized rice bran and oat bran lower cholesterol in humans F.A.S.E.B. J. 4: A 368
Hegsted M, Windhauser MM, Morris K & Lester SB . 1993 Stabilized rice bran and oat bran lower cholesterol in humans Nutrition Research 13: 387–298
Holland B, Welch AA, Unwin ID, Buss DH, Paul AA & Southgate DAT . 1991 McCance & Widdowson's The Composition of Foods, 5th Edition. Royal Society of Chemistry: Cambridge
Homocysteine Lowering Trialists' Collaboration . 1998 Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials Br. Med. J. 316: 894–898
Jacques PF, Selhub J, Boston AG, Wilson PWF & Rosenberg IH . 1999 The effect of folic acid fortification on plasma folate and total homocysteine concentrations New Engl. J. Med. 340: 1449–1454
Jacobs DR Jr, Meyer KA, Kushi LH & Folsom AR . 1998 Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: the Iowa Women's Health Study Am. J. Clin. Nutr. 68: 248–257
Jacobs DR Jr, Meyer KA, Kushi LH & Folsom AR . 1999 Is whole-grain intake associated with reduced total and cause-specific death rates in older women? The Iowa Women's Health Study Am. J. Public Health 89: 322–329
Jacobs DR, Meyer HE & Solvoll K . 2001 Reduced mortality among whole grain bread eaters in men and women in the Norwegian County Study Eur. J. Clin. Nutr. 55: 137–143
Jenkins DJA, Hill MS, Cummings JH . 1975 Effect of wheat fiber on blood lipids, fecal steroid excretion and serum iron Am. J. Clin. Nutr. 28: 1408–1411
Jenkins DJA, Wesson V, Wolever TMS, Jenkins AL, Kalmusky J, Giudici S, Csima A, Josse RG & Wong GS . 1988 Wholemeal versus wholegrain breads: proportion of whole or cracked grain and the glycaemic response Br. Med. J. 297: 958–960
Joint FAO/WHO Expert Consultation, Rome 14–18 April, 1997 . 1998 Carbohydrates in Human Nutrition FAO Food & Nutrition Paper 66, Rome: FAO
Judd PA & Truswell AS . 1981 The effect of rolled oats on blood lipids and fecal steroid excretion in man Am. J. Clin. Nutr. 34: 2061–2067
Kay RM & Truswell AS . 1977 The effect of wheat fiber on plasma lipids and faecal steroid excretion in man Br. J. Nutr. 37: 227–235
Kashtan H, Stern HS, Jenkins DJA, Jenkins AL, Hay K, Marcon N, Minkin S & Bruce WR . 1992 Wheat-bran and oat-bran supplements' effects on blood lipids and lipoproteins Am. J. Clin. Nutr. 55: 976–980
Keenan JM, Wenz JB, Myers S, Ripsin C & Huang Z . 1991 Randomized, controlled, crossover trial of oat bran in hypercholesterolemic subjects J. Family Practice 33: 600–608
Kestin M, Moss R, Clifton PM & Nestel PJ . 1990 Comparative effects of three cereal brans on plasma lipids, blood pressure and glucose metabolism in mildly hypercholesterolemic men Am. J. Clin. Nutr. 52: 661–666
Khaw KT & Barrett-Connor E . 1987 Dietary fiber and reduced heart disease mortality rates in men and women: a 12 y prospective study Amer. J. Epidemiol. 126: 1092–1103
Kirby RW, Anderson JW, Sieling B, Rees ED, Chen WJL, Miller RE & Kay RM . 1981 Oat-bran intake selectively lowers serum low-density lipoprotein cholesterol concentrations of hypercholesterolemic men Am. J. Clin. Nutr. 34: 824–829
Knekt P, Reunaen A, Järvinen R, Seppänen R, Heliövaara M & Aroura A . 1994 Antioxidant vitamin intake and coronary mortality in a longitudinal population study Am. J. Epidemiol. 139: 1180–1189
Kretsch MJ, Crawford L & Calloway DH . 1979 Some aspects of bile acid and urobilinogen excretion and faecal elimination in men given a rural Guatemalan diet and egg formula with and without oat bran Am. J. Clin. Nutr. 32: 1492–1496
Kromhout D, Bosschieter EB & de Lezenne Coulander C . 1982 Dietary fibre and 10 y mortality from coronary heart disease, cancer and all causes Lancet ii: 518–522
Lagiou P, Trichopoulou A, Hendrickx HK, Kelleher C, Leonhauser IU, Moreiras P, Nelson M, Schmitt A, Sekula W, Trygg K & Zajkas G . 1999 Household budget survey nutritional data in relation to mortality from coronary heart disease, colorectal cancer and female breast cancer in European countries Eur. J. Clin. Nutr. 53: 328–332
Leadbetter J, Ball MJ & Mann JI . 1991 Effect of increasing quantities of oat bran in hypercholesterolemic people Am. J. Clin. Nutr. 54: 841–845
Lepre F & Crane S . 1992 Effect of oatbran on mild hyperlipidaemia Med. J. Aust. 157: 305–308
Lindegärde F & Larsson L . 1984 Effect of a concentrated bran fibre preparation on HDL-cholesterol in hypercholesterolaemic men Human Nutr. Clin. Nutr. 38C: 39–45
Lui S, Stampfer MJ, Hu FB, Giovanucci E, Rimm E, Manson JE, Hennekens CH & Willett WC . 1999 Whole grain consumption and risk of coronary heart disease: results from the Nurses' Health study Am. J. Clin. Nutr. 70: 412–419
Luyken R, de Wijn JF, Pikaar NA & van der Meer R . 1965 De invloed van havermout op het serum cholesterolgehalte van het bloed Voeding 26: 229–244
Mackay S & Ball MJ . 1992 Do brans and oatbran add to the effectiveness of a low fat diet? Eur. J. Clin. Nutr. 9: 641–648
McCance RA & Widdowson EM . 1956 Breads White and Brown. Their Place in Thought and Social History Pitman Medical: London
McDougall RM, Yakymyshyn L, Walker K & Thurston OG . 1978 Effect of wheat bran on serum lipoproteins and biliary lipids Can. J. Surg. 21: 433–435
McIntosh GM, Whyte J, McArthur R & Nestel PJ . 1991 Barley and wheat foods: influence on plasma cholesterol concentrations in hypercholesterolemic men Am. J. Clin. Nutr. 53: 1205–1209
McLennan W & Podger A . 1997 National Nutrition Survey Selected Highlights Australia 1995 Australian Bureau of Statistics, Department of Health: Canberra
Marr JW . 1971 Individual dietary surveys: purposes and methods World Review of Nutrition & Dietetics 13: 106–164
Ministry of Agriculture, Fisheries & Food . 1994 The Dietary and Nutritional Survey of British Adults Further Analysis HMSO: London
Morris JN, Heady JA, Raffle PAB & Parks JW . 1953 Coronary heart disease and physical activity of work Lancet ii: 1053–1057–1111–1120
Morris JN, Marr JW & Clayton DG . 1977 Diet and heart: A postscript Br. Med. J. 2: 1307–1314
Mugford DC, Griffith PJ & Walker AR . 1996 Nutrient levels in white, mixed grain and wholemeal bread. An Australia-wide survey of breads from different bakeries and different States. Food Australia 48: 264–269
Munoz JM, Sandstead HH & Jacobs RA . 1979 Effect of some cereal brans and TVP on plasma lipids Am. J. Clin. Nutr. 32: 580–592
National Health & Medical Research Council. 1992 Dietary Guidelines for Australians Australian Government Publishing Service: Canberra
National Health & Medical Research Council. 1999 The prevention, early detection and management of colorectal cancer Clinical Practice Guidelines NH & MRC: Canberra
Newman RK, Lewis SE, Newman CW, Boik RJ & Ramage RT . 1989 Hypercholesterolemic effect of barley foods on healthy men Nutr. Rep. Int. 39: 749–759
Normand FL, Ory RL & Modd RR . 1987 Binding of bile acids and trace minerals by soluble hemicelluloses of rice Food Technol. 41: 86–99
O'Moore RR, Flanagan M, McGill AR, Wright EA, Little C & Weir DG . 1978 Diet and heart disease Br. Med. J. i: 1213
Pietinen P, Rimm EB, Korhonen P, Hartman AM, Willett WC, Albanes D & Virtamo J . 1996 Intake of dietary fiber and risk of coronary heart disease in a cohort of Finnish men: the Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Circulation 94: 2720–2727
Poulter N, Chang CL, Cugg A, Poulter C, Sever P & Thom S . 1993 Lipid profiles after the daily consumption of an oat-based cereal: a controlled crossover trial Am. J. Clin. Nutr. 58: 66–69
Raymond TL, Connor WE, Lin DS, Warner S, Fry MM & Connor SL . 1977 The interaction of dietary fibers and cholesterol upon the plasma lipids and lipoproteins, sterol balance and bowel function in human subjects J. Clin. Invest. 60: 1429–1437
Reynolds HR, Lindeke E & Hunninghake DB . 1989 Effect of oat bran on serum lipids J. Am. Diet. Ass. 89 Suppl: A112
Rimm EB, Ascherio A, Giovanucci E, Spiegelman D, Stampfer MJ & Willett WC . 1996 Vegetable, fruit and cereal fiber intake and risk of coronary heart disease among men J.A.M.A. 275: 447–541
Roth G & Leitzmann C . 1985 Langezeiteinfluss ballaststoffereicher Frühstückscerealien auf die Blutlipide beim Menschen Akt. Ernähr. 10: 106–109
Salt Skip News . 1994, August Kelloggs still reducing salt in breakfast cereals Menzies Centre for Population Health Research: Hobart
Sanders TAB & Reddy S . 1991 The influence of rice bran on plasma lipids and lipoproteins in human volunteers Eur. J. Clin. Nutr. 46: 161–172
Saudia TL, Barfield BR & Barger J . 1992 Effect of oat bran consumption on total cholesterol levels in healthy adults Mil. Med 157: 567–568
Select Committee on Nutrition and Human Needs, United States Senate . 1977 Dietary Goals for the United States US Government Printing Office: Washington DC
Slavin JL, Martini MC, Jacobs DR & Marquart L . 1999 Plausible mechanisms for the protectiveness of whole grains Am. J. Clin. Nutr. 70: 459S–463S
Spencer H, Norris C, Derler J & Osis D . 1991 Effect of oat bran muffins on calcium absorption and calcium, phosphorus, magnesium and zinc balance in men J. Nutr. 121: 1976–1983
Stasse-Wolthuis M, Katan MN, Hermus RJJ, Hautvast JGAJ . 1979 Increase of serum cholesterol in men fed a bran diet Atherosclerosis 45: 87–91
Stasse-Wolthuis M, Albers HFF, van Jeveren JGC et al. 1980 Influence of dietary fiber from vegetables and fruits, bran or citrus pectin on serum lipids, fecal lipids and colonic function Am. J. Clin. Nutr. 33: 1745–1756
Stephen AM, Sieber GM, Gerster YA & Morgan DR . 1995 Intake of carbohydrate and its components-international comparisons, trends over time, and effects of changing to low-fat diets Am. J. Clin. Nutr. 62: 815S–867S
Stewart FM, Neutze J & Newsome-White R . 1992 The addition of oatbran to a low fat diet has no effect on lipid values in hypercholesterolaemic subjects N.Z. Med. J. 105: 398–400
Storch K, Anderson JW & Young VR . 1984 Oat-bran muffins lower serum cholesterol of healthy young people Clin. Res. 34: 740A
Swain JF, Rouse IL, Curley CB & Sacks FM . 1990 Comparison of the effects of oat bran and low-fibre wheat on serum lipoprotein levels and blood pressure New Engl. J. Med. 322: 147–152
Thompson LU . 1993 Potential health benefits and problems associated with antinutrients in foods Food Research International 26: 131–149
Tietyen JL, Nevins DJ & Schneeman BO . 1990 Characterization of the hypercholesterolemic potential of oat bran F.A.S.E.B. J. 4: A527
Törrönen R, Kansanen L, Uusitupa M, Hänninen O, Myllmäki O, Härkönen H & Mälkki Y . 1992 Effect of an oat bran concentrate on serum lipids on free-living men with mild to moderate hypercholesterolaemia Eur. J. Clin. Nutr. 9: 621–627
Trowell H . 1975 Coronary heart disease and dietary fiber Am. J. Clin. Nutr. 28: 798–800
Truswell AS & Beynen AC . 1992 Dietary fibre and plasma lipids: potential for prevention and treatment of hyperlipidaemias. In Dietary Fibre—a Component of Food Nutrition functions in Health and Disease, TF Schweizer & CA Edwards (eds). 295–332 ILSI Human Nutrition Reviews. Springer Verlag: London
Truswell AS & Kay RM . 1976 Bran and blood lipids Lancet i: 367
Truswell AS . 1995 Dietary fibre and blood lipids Current Opinion in Lipidology 6: 14–19
Truswell AS, Morris, Sinclair, Low-Beer, McLean-Baird, Heaton, Eastwood & Trowell . In Discussion after paper by JN Morris, JW Marr & DG Clayton: In KW Heaton (ed). 1978 Dietary Fibre 3rd Kellogg Nutrition symposium 54–56 London: Newman Publishing.
Turnbull WH & Leeds AR . 1987 Reduction of total and LDL-cholesterol in plasma by rolled oats J. Clin. Nutr. Gastroenterol. 2: 177–181
Van dokkum W . 1978 Zemelen in brood: verteerbaarheid en invloed op het defaecatiepatroon, de mineralen-balans en de serumlipidconcentraties bij de mens Voedingsmiddelentechnologie 11: 18–21
Van Horn L, Lui K, Parker D, Emidy L, Liao Y, Pan WH, Giumetti D, Hewitt J & Stampler J . 1986 Serum response to oat product intake with a fat-modified diet J. Am. Diet. Ass. 86: 759–764
Van Horn L, Emidy L, Lui K, Liao Y, Ballew C, King J & Stanley J . 1988 Serum lipid responses to fat-modified oatmeal-enhanced diet Prev. Med. 17: 377–386
Van Horn L, Moag-Stahlberg A, Lui K, Ballew C, Ruth K, Hughes R & Stamler J . 1991 Effects on serum lipids of adding instant oats to usual American diets Am. J. Pub. Hlth. 81: 183–188
Welch RW, Peterson DM & Schranka N . 1988 Hypercholesterolemic and gastrointestinal effects of oat bran fractions in chicks Nutr. Rep. Int. 38: 551–561
Whyte JL, McArthur R, Topping D & Nestel P . 1992 Oat bran lowers plasma cholesterol levels in mildly hypercholesterolemic men J. Am. Diet. Ass. 92: 446–449
Willett WC . 1998 The dietary pyramid: does the foundation need repair? Am. J. Clin. Nutr. 68: 218–219
Wolk A, Manson JE, Stampfer MJ, Colditz GA, Hu FB, Speizer FE, Hennekens CH & Willett WC . 1999 Long-term intake of dietary fiber and decreased risk of coronary heart disease among women J.A.M.A. 281: 1998–2004
Yoshino G, Kazumi T, Amaino M, Tateiwa M, Yamasaki T, Takashima S, Iwai M, Hatanaka H & Baba S . 1989 Effects of gamma-oryzanol on hyperlipidemic subjects Curr. Ther. Res. Clin. Experience 45: 543–552
Zhang J-X, Hallman SG, Andersson H, Bosaeus I, Åman P, Tidehag P, Stenling R, Lundin E, Dahlgren S . 1992 Effect of oat bran on plasma cholesterol and bile acid excretion in nine subjects with ileostomies Am. J. Clin. Nutr. 56: 99–105
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Truswell, A. Cereal grains and coronary heart disease. Eur J Clin Nutr 56, 1–14 (2002). https://doi.org/10.1038/sj.ejcn.1601283
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601283
Keywords
This article is cited by
-
Ganoderma lucidum polysaccharide ameliorates cholesterol gallstone formation by modulating cholesterol and bile acid metabolism in an FXR-dependent manner
Chinese Medicine (2024)
-
Mitigation of heat stress in wheat (Triticum aestivum L.) via regulation of physiological attributes using sodium nitroprusside and gibberellic acid
BMC Plant Biology (2023)
-
Effect of drying technique on physiochemical and nutritional properties of Eleusine coracana (finger millet) porridge powder
Journal of Food Science and Technology (2023)
-
Cereal intake and mortality in older Chinese: a 15-year follow-up of a prospective cohort study
European Journal of Nutrition (2023)
-
The consumption of wholegrain is related to depressive symptoms among Chinese adults: a cross-sectional study
European Journal of Clinical Nutrition (2022)