Abstract
Data sources
Cochrane Oral Health's Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), Medline Ovid, Embase, Ovid, CancerLit via PubMed. MetaRegister of Controlled Trials, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform were searched for ongoing trials.
The reference lists of included studies and existing reviews were manually searched.
Study selection
Randomised control trials (RCTs) including patients diagnosed with oral leukoplakia comparing any treatment to placebo or no treatment were included with no restrictions on language or date of publication.
Data extraction and synthesis
Two reviewers independently checked for inclusion, performed data extraction using a specially designed form, and assessed the risk of bias for each study and by domain over all studies. The primary outcome considered was onset of oral cancer reported as dichotomous data. Secondary outcomes were clinical resolution, histological changes and adverse events that were mostly reported as ordinal data.
Results
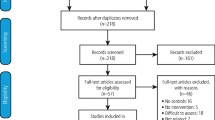
Fourteen studies with 909 participants were included in the review. Of the included studies, four compared topical interventions to placebo, nine compared systemic interventions to placebo, and one compared a combination of topical and systemic treatments versus placebo. The risk of bias was considered to be low in one study, unclear in seven, and high for the remaining six. Only three studies provided usable data on the primary outcome: cancer incidence. Clinical improvement was achieved in three studies using: systemic vitamin A or retinoids (two studies) and systemic beta-carotene or carotenoids (one study). Only two studies using beta-carotene or carotenoids were meta-analysed, showing no benefit on the outcome cancer development.
Conclusions
There is no evidence that any of the active treatments included work better than placebo in reducing the risk of developing oral cancer.
Similar content being viewed by others
Commentary
This is an update of the 2006 review, which performed a comprehensive search of published and ongoing RCTs comparing any treatment to placebo or no treatment of oral leukoplakia until May 2016.
This quality review included 14 studies with 909 participants. Of those 14, four studies used topical interventions comparing different agents to placebo (129 participants). Of the systemic interventions, five compared a single intervention against placebo (329 participants), while three compared a different strength of the same agent to placebo (173 participants) and one compared two agents to placebo (131 participants). Results were combined for the studies with multiple arms investigating different strengths of the same agent.
The interventions were grouped into: vitamin A or retinoids (four studies: three systemic), beta-carotene and carotenoids (three systemic studies), non-steroidal anti-inflammatory drugs (two studies), herbal extracts (four studies: one topical, two systemic, one both), bleomycin (one topical study) and Bowman-Birk Inhibitor (one systemic study).
The inconclusive nature of this quality review highlights gaps in the knowledge and limitations of the current evidence. While leukoplakia is considered an oral premalignant lesion, the non-homogeneous variety carries a higher risk of malignant transformation compared to its homogeneous counterpart, and histological characteristics are variable for both forms, independently of the clinical appearance; therefore the variability of the histological and clinical type of the participants included in each of the studies is not surprising, although under the umbrella of leukoplakia, studies included histological grading varying from hyperplasia all the way to carcinoma in situ.
The rate of malignant transformation of leukoplakia is still a subject of debate. There is indication that it may range from as low as 0.13% to as high as 34%. The risk increases depending on some clear risk factors: location (tongue and floor of the mouth), appearance (non-homogeneous), size (larger than 2 cm), sex of patient (female), and being a non-smoker. Stratification of the results based on the initial histological presentation and the known risk factors may provide more insightful information.
The choice of oral cancer onset as primary outcome, although ideal, may not be easily attainable when one considers that there is limited information on the rate of malignant transformation, and even less on timing. As a result, the duration of studies needs careful consideration to help address this and other important issues like safety and long-term effectiveness, as those are vital attributes of any chemo-preventive agent.
The use of changes in clinical size and clinical resolution as a surrogate, although widely accepted, may not always correlate to changes at the cellular level. Multiple studies include changes in different biologic markers. Molecular abnormalities might hold the key for better predicting oncogenic potential in the near future.
Sample size of the included studies has to be taken into consideration, since the smaller studies might not have enough power to detect a difference. Designing studies with adequate sample size can be challenging because of fundamental unknowns on the malignant conversion.
At the present time, we know that leukoplakia is a clinical diagnosis that warrants histological investigation and adequate follow-up.
Author information
Authors and Affiliations
Additional information
Address for correspondence: Luisa Fernandez Mauleffinch, Review Group Co-ordinator, Cochrane Oral Health Group, MANDEC, School of Dentistry, University of Manchester, Higher Cambridge Street, Manchester, M15 6FH, UK. E-mail: luisa.fernandez@manchester.ac.uk
Lodi G, Franchini R, Warnakulasuriya S, Varoni EM, Sardella A, Kerr AR, Carrassi A, MacDonald LC, Worthington HV. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev 2016; 7: CD001829. doi: 10.1002/14651858.CD001829.pub4.
This paper is based on a Cochrane Review published in the Cochrane Library 2016, issue 7 (see www.thecochranelibrary.com for information). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and the Cochrane Library should be consulted for the most recent version of the review.
Rights and permissions
About this article
Cite this article
Spivakovsky, S. Limited evidence for interventions to treat oral leukoplakia. Evid Based Dent 18, 92–93 (2017). https://doi.org/10.1038/sj.ebd.6401261
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6401261